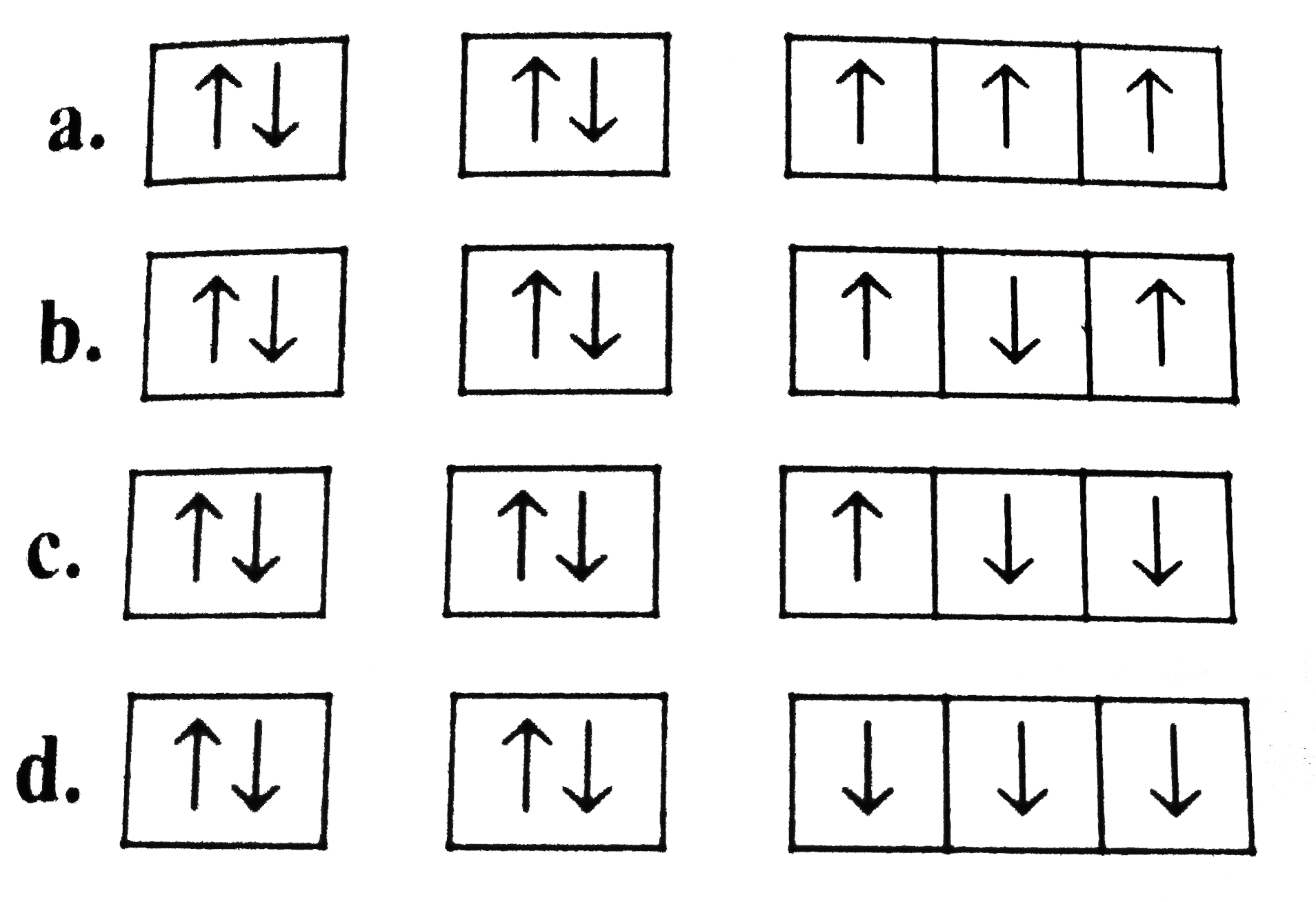
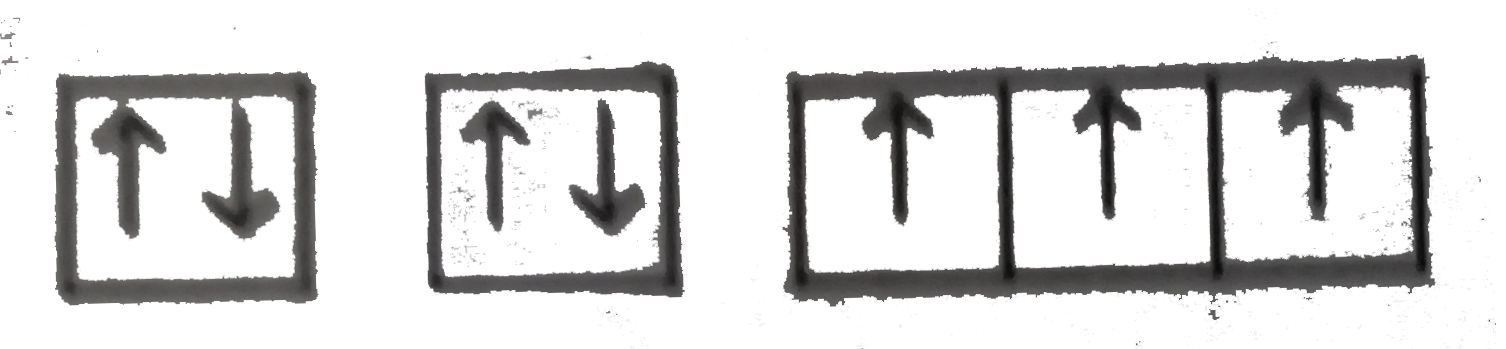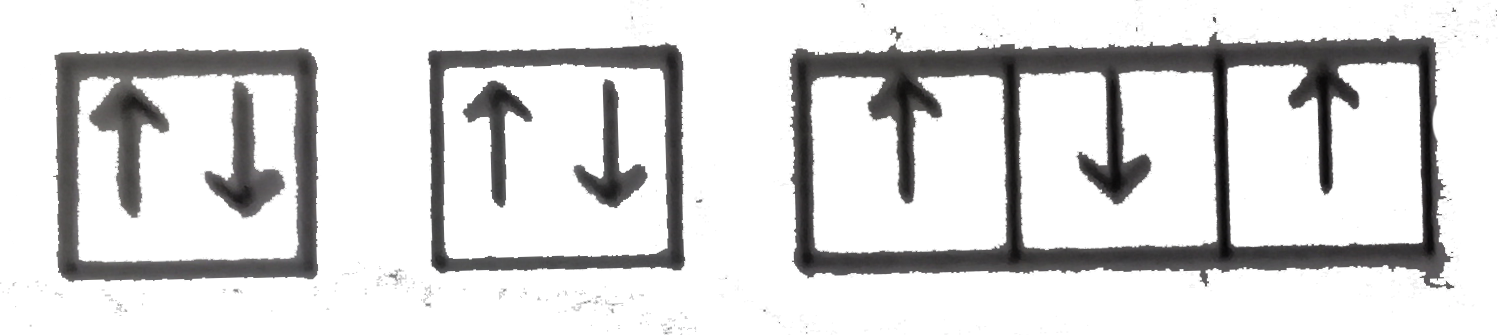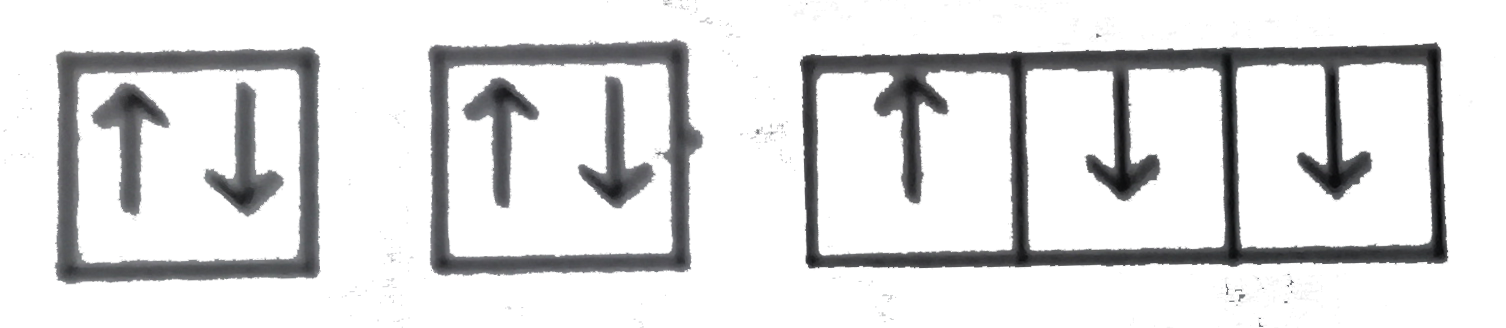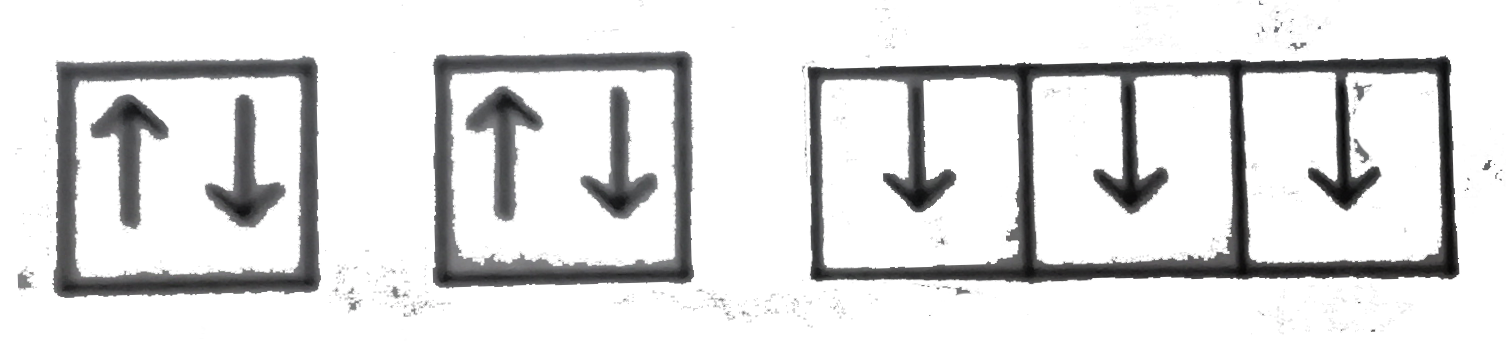A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|127 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion And Reason|21 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|50 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY ENGLISH|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|15 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ATOMIC STRUCTURE-Exercises Multiple Correct
- Which of the following statement are correct ?
Text Solution
|
- The ground state electronic configeration of nitrogen atom can be re...
Text Solution
|
- Which of the following orbital has (have) one spherical node?
Text Solution
|
- The energy of an electron in the first level of H atom is - 13.6 eV ...
Text Solution
|
- Which of the following species has (have) five unpaired electron ?
Text Solution
|
- Which of the following series in H-spectra occurs in IR region
Text Solution
|
- Which of the following elements are isotopes
Text Solution
|
- Which of the following properies by cathode ray?
Text Solution
|
- Which of the following are isotones ?
Text Solution
|
- The energy of an electron in the first Bohr orbit of H atom is -13.6 e...
Text Solution
|
- When alpha particle are sent through a thin metal foil ,most of the...
Text Solution
|
- Which of the following sets of quantum number is //are not perrmitted...
Text Solution
|
- The lightest particle is/are
Text Solution
|
- Which orbit of the following is lower in energy in a many electron at...
Text Solution
|
- Which of the following statement (s) is/are correct ?
Text Solution
|
- The angular momentum of d electron is
Text Solution
|
- The angular momentum of p electron is
Text Solution
|
- Which of the following ie//are posssible ?
Text Solution
|
- If the value of (n + l) is more than 3 and less than 6 , then what wi...
Text Solution
|
- Which of the following is//are not indicated by the sign of lobes in ...
Text Solution
|