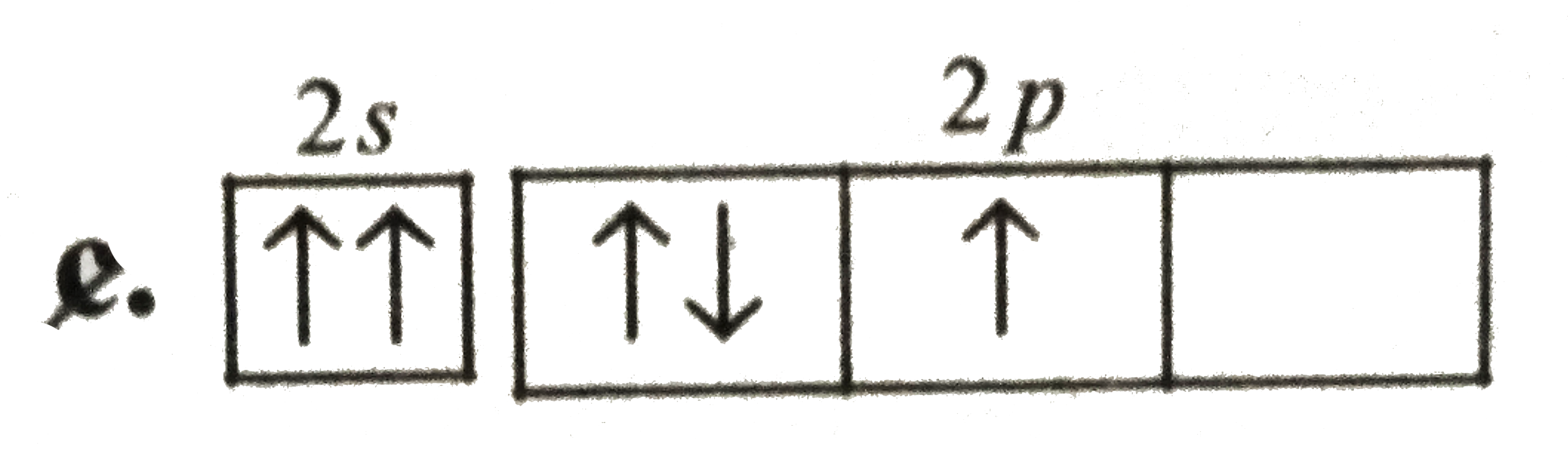
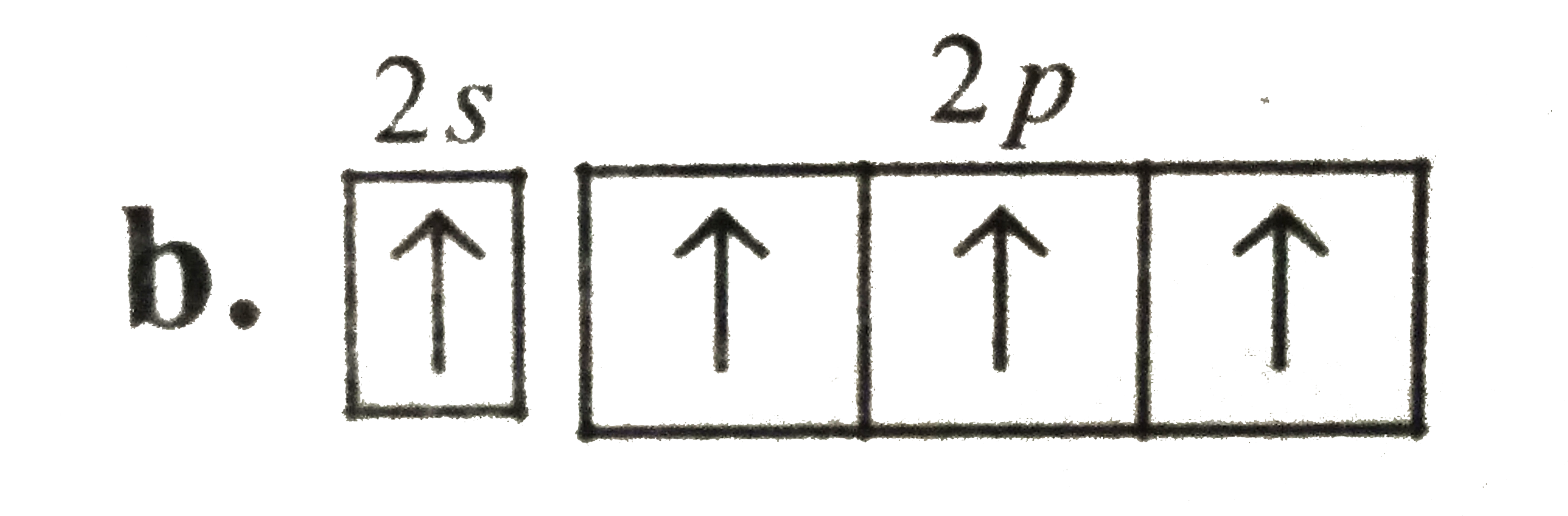A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion And Reason|21 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Integer|11 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|45 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY ENGLISH|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|15 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ATOMIC STRUCTURE-Exercises Single Correct
- Bohr's model of atom is not in agrement with
Text Solution
|
- If the energy of electron in H atom is given by expression - 1312 n^(...
Text Solution
|
- For which of the following electron distribution in ground state the P...
Text Solution
|
- Which of the following orbital does not make sense?
Text Solution
|
- Which of the following sets of quantum number is not possible
Text Solution
|
- The possible sub-shell in n = 3 energy shell are
Text Solution
|
- In the Schrodingers wave equation spi repressents
Text Solution
|
- Heisenberg's uncertainty principal rules out the exact simultaneous ...
Text Solution
|
- The two electron have the following sets of quantum number X 3,2 ...
Text Solution
|
- When electronic transition occurs from higher energy state to lowe...
Text Solution
|
- Which of the following statement concerning Bohr's model is false ?
Text Solution
|
- Which of the following gave the idea of nucless of the atom ?
Text Solution
|
- A body of mass 10 g is moving with a velocity of 100ms^(-1). The wavel...
Text Solution
|
- Name a series of lines of hydrogen spectrum which lies in : (1) Visibl...
Text Solution
|
- The transitionis He^(o+) ion that would have the same wavelength a...
Text Solution
|
- The photoelectric work - function of potassium is 2.3 eV. If light h...
Text Solution
|
- A certain metal when irradiated by light (v=3.2xx10^(16)Hz) emits phot...
Text Solution
|
- The number of spherical nodes in 3p-orbital is/are
Text Solution
|
- Which of the following orbitals does not have the angular node ?
Text Solution
|
- The ratio of the first three Bohr orbit radii is
Text Solution
|