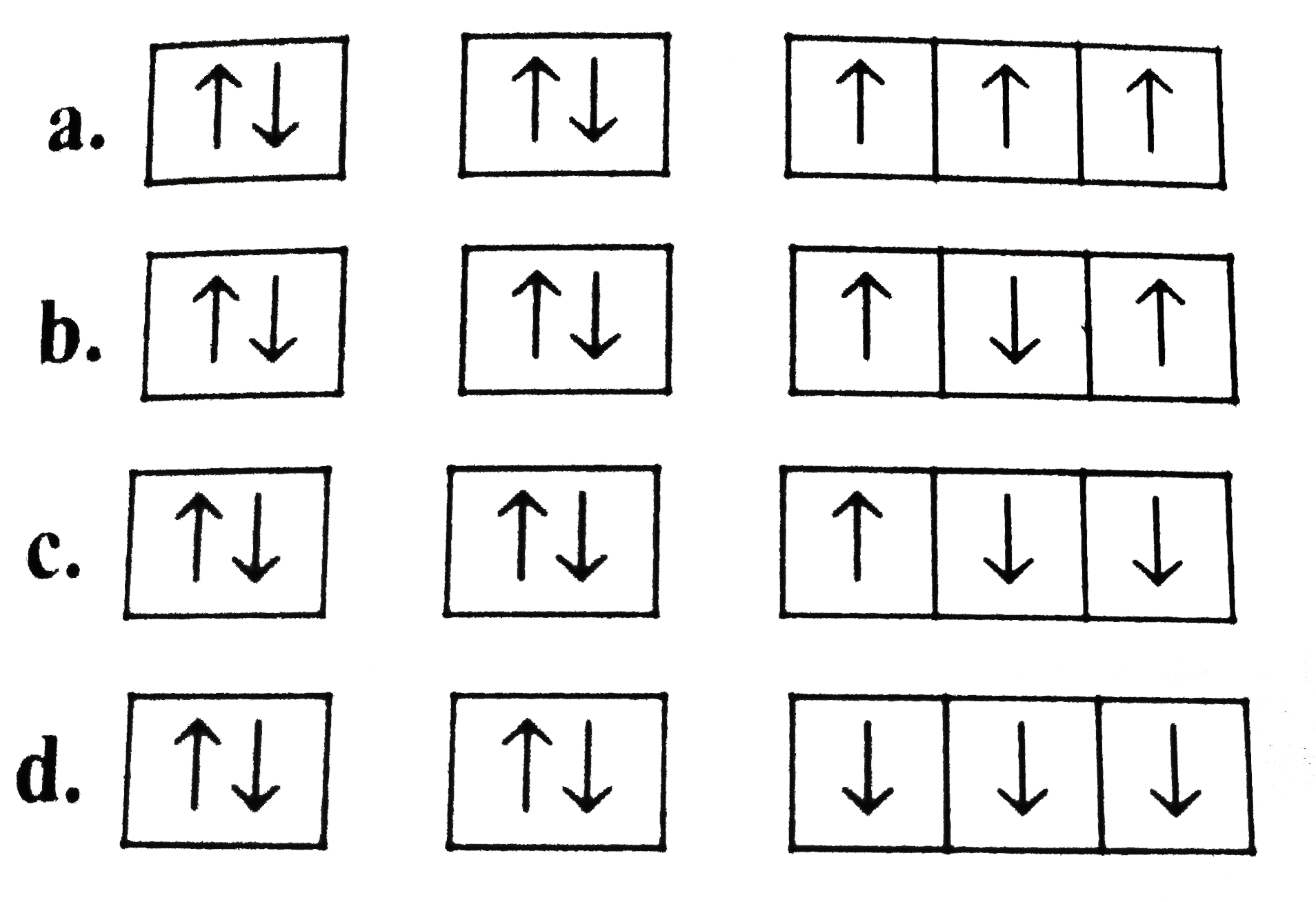
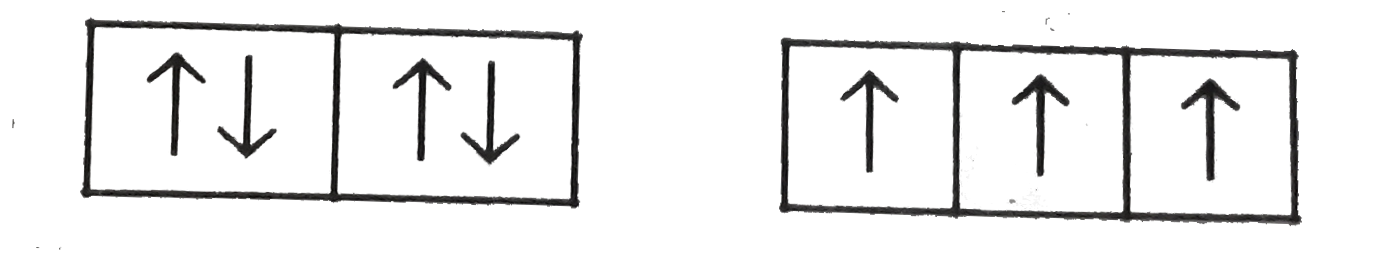
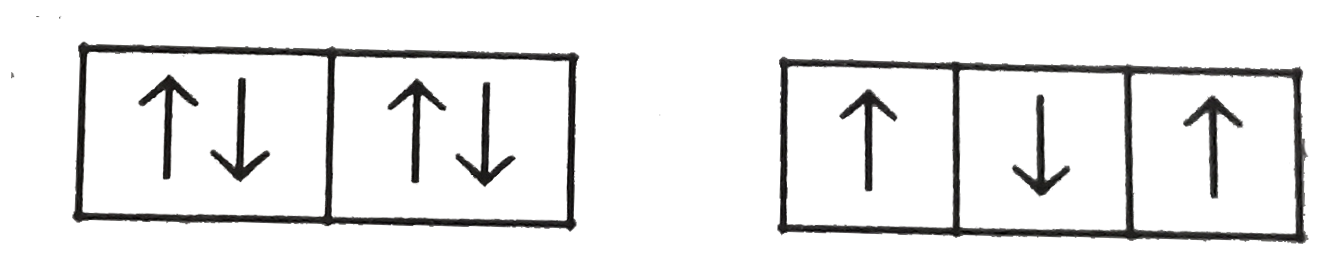
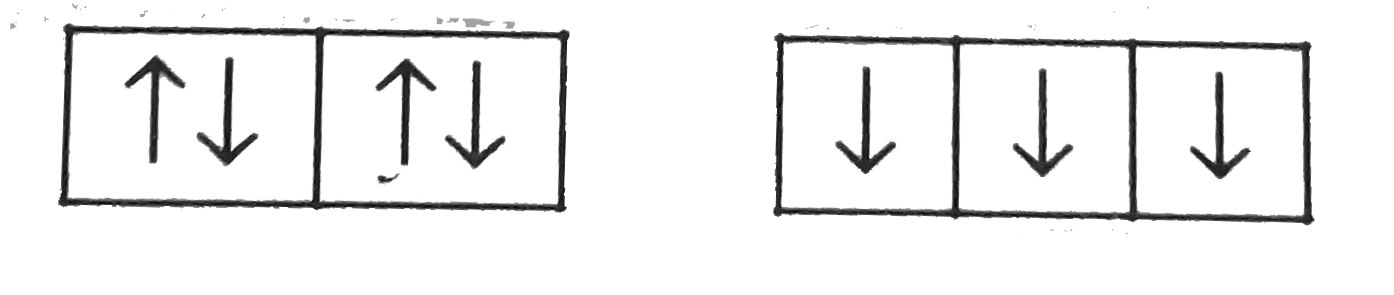
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Single Correct|36 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Integer|2 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Linked Comprehension|3 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY ENGLISH|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|15 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ATOMIC STRUCTURE-Archives Multiple Correct
- The isotone (s) of .(32)^(77)Ge is//are
Text Solution
|
- When alpha particle are sent through a this metal foil mass of then g...
Text Solution
|
- Many elements have non-integral atomic masses because
Text Solution
|
- The sum of the number of neutrons and protons in the isotopes of hydro...
Text Solution
|
- The atomic nucleus contaits
Text Solution
|
- Which of the following statement are correct ?
Text Solution
|
- The ground state electronic configeration of nitrogen atom can be re...
Text Solution
|