A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Integer|2 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Fill In The Balnks|8 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Multiple Correct|7 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY ENGLISH|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|15 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ATOMIC STRUCTURE-Archives Single Correct
- The principal quantum number of an atom is related in the
Text Solution
|
- Which electronic level would allow the hydrogen atom to absorb a photo...
Text Solution
|
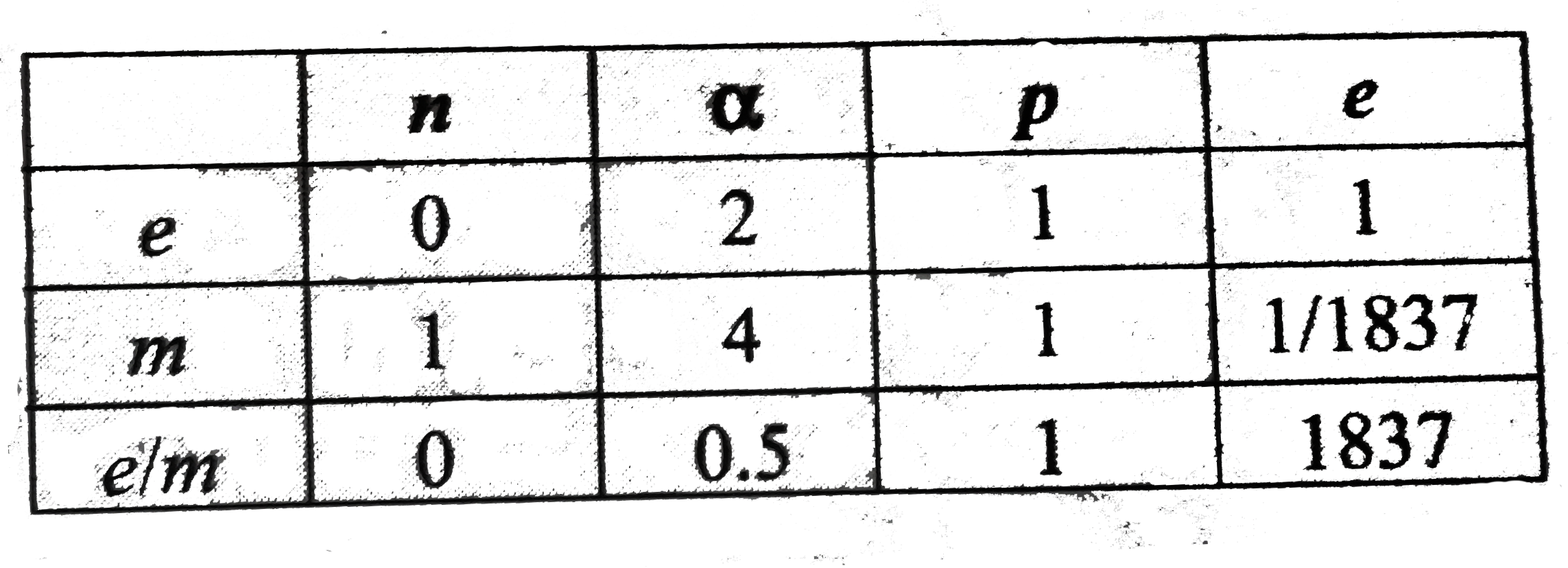
- The increasing order (lowest first) for the values of e//m (charge/mas...
Text Solution
|
- The correct set of four quantum numbers for the valence elections of r...
Text Solution
|
- Of the following the radition having the maximum wavelength is
Text Solution
|
- Bohr's model can explain
Text Solution
|
- The radius of an atomic nucleus is of the order of
Text Solution
|
- Rutherford's alpha particle scattering experiment eventually led to t...
Text Solution
|
- Which of the following sets of quantum numbers represents an imposs...
Text Solution
|
- The ratio of energy of photon of lambda = 2000 Å to that of lambda = 4...
Text Solution
|
- The wavelngth fo a spectrl line for an electronic transition is invers...
Text Solution
|
- The triad of nuclie that are isotomic is
Text Solution
|
- The orbital diagram in which the Aufbau principle is violated is
Text Solution
|
- The outermost electric configuration of the most electron of chlorine...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of chlori...
Text Solution
|
- The correct ground state electronic configuration of chromium atom is
Text Solution
|
- Which of the following does not characterise X-rays?
Text Solution
|
- Which of the following relates to photons both as wave motion and as a...
Text Solution
|
- Which of the following has the maximum number of ampaired electrons ?
Text Solution
|
- What will be the orbital angular momentum of an electron in 2s-orbital...
Text Solution
|