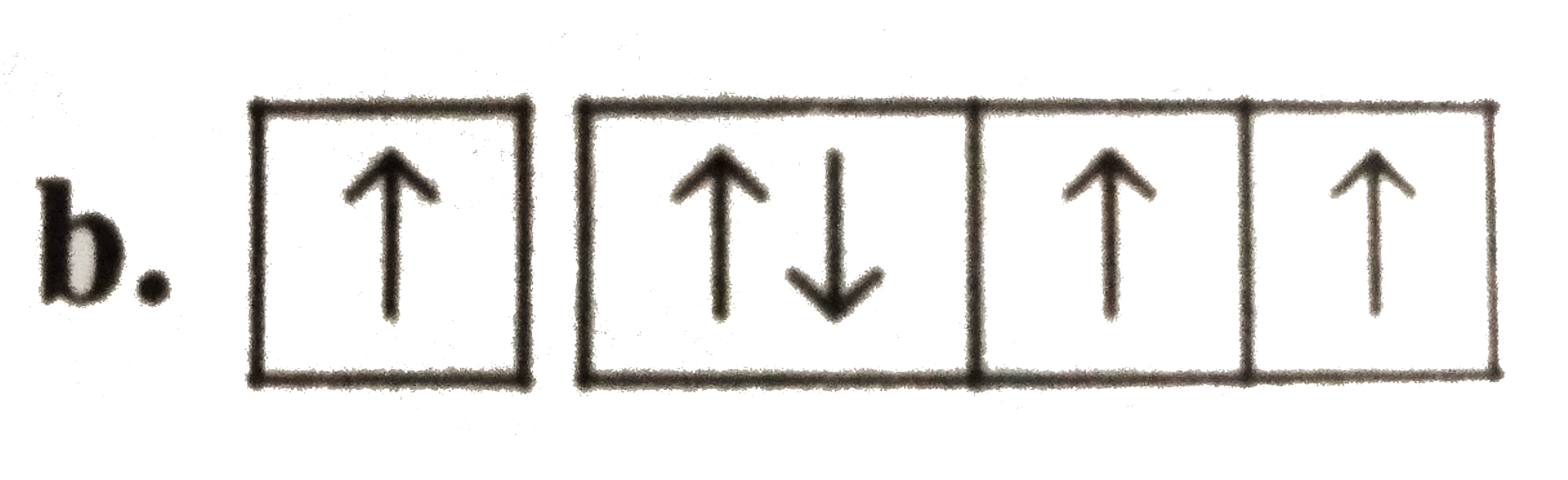
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Integer|2 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Fill In The Balnks|8 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Multiple Correct|7 VideosAPPENDIX - INORGANIC VOLUME 1
CENGAGE CHEMISTRY ENGLISH|Exercise chapter-7 Single correct answer|1 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|15 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ATOMIC STRUCTURE-Archives Single Correct
- The wavelngth fo a spectrl line for an electronic transition is invers...
Text Solution
|
- The triad of nuclie that are isotomic is
Text Solution
|
- The orbital diagram in which the Aufbau principle is violated is
Text Solution
|
- The outermost electric configuration of the most electron of chlorine...
Text Solution
|
- The correct set of quantum numbers for the unpaired electron of chlori...
Text Solution
|
- The correct ground state electronic configuration of chromium atom is
Text Solution
|
- Which of the following does not characterise X-rays?
Text Solution
|
- Which of the following relates to photons both as wave motion and as a...
Text Solution
|
- Which of the following has the maximum number of ampaired electrons ?
Text Solution
|
- What will be the orbital angular momentum of an electron in 2s-orbital...
Text Solution
|
- The first use of quantum theory to explain the structure of atom was m...
Text Solution
|
- For a d electron the orbital angular momentum is
Text Solution
|
- The energy of an electron in the first Bohr orbit of H atom is -13.6 e...
Text Solution
|
- The electrons identified by the following quantum numbers n and l: (i)...
Text Solution
|
- The electronic configuration of an element is 1s^(2)2s^(2)2p^(6)3s^(2)...
Text Solution
|
- The de Broglie wavelength associated with a ball of mass 200 g and mov...
Text Solution
|
- The number of nodes palnes in a p(y) orbital is
Text Solution
|
- The quantum number + 1//2 and -1//2 for the electron spin represent
Text Solution
|
- Rutherford's experiment , which established the nuclear model of the ...
Text Solution
|
- If nitrogen atoms had electronic configuration 1s7 It would have ener...
Text Solution
|