A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Illustration|2 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises|21 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|11 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|33 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-STATES OF MATTER-Exercises (Ture False)
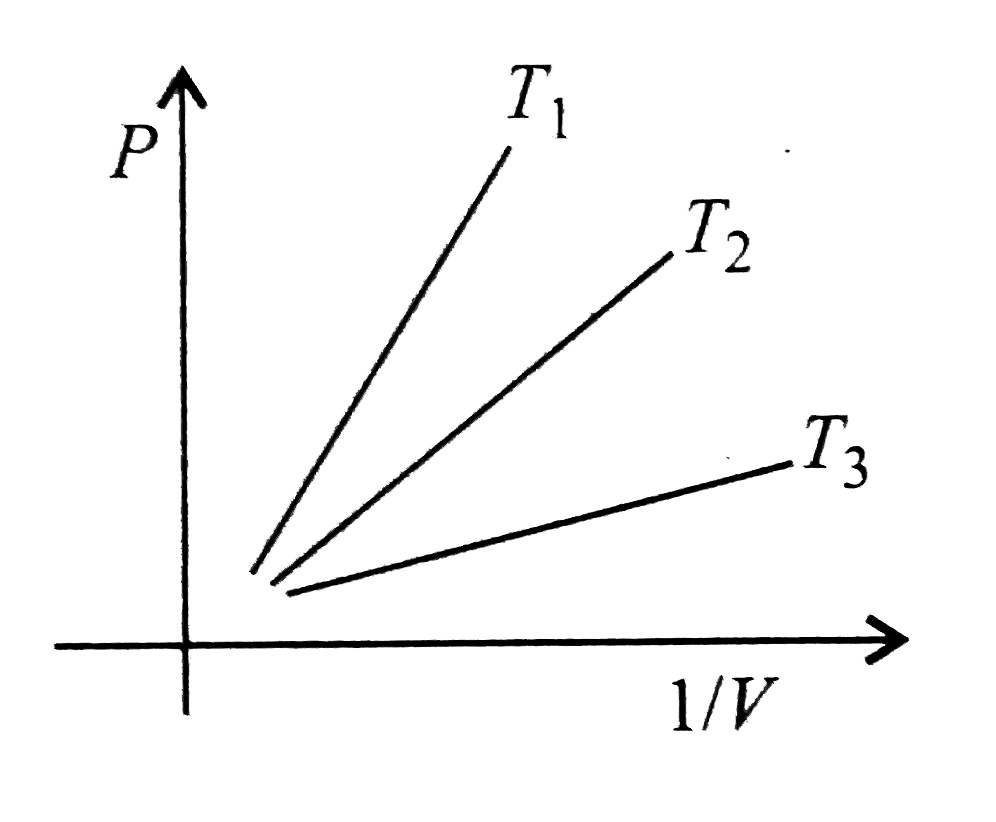
- The curve drawn below shows the variations of P as a function of 1//V ...
Text Solution
|
- In the van der Waals equation (P + (n^(2)a)/(V^(2)))(V - nb) = nRT ...
Text Solution
|
- Kinetic energy of a molecule is zero at 0^(@)C
Text Solution
|
- Gas in a closed container will exert much higher pressure due to gravi...
Text Solution
|
- The graph between PV vs P at constant temperature is linear parallel t...
Text Solution
|
- Real gases show deviation from ideal behavior at low temperature and h...
Text Solution
|
- All the molecules in a given sample of gas move with same speed.
Text Solution
|
- Small value of a means, gas can be easily liqueifed.
Text Solution
|
- Small value of a means, gas can be easily liqueifed.
Text Solution
|
- Rate of diffusion is directly proportional to the square root of molec...
Text Solution
|
- For ideal gases, Z = 1 at all temperature and pressure.
Text Solution
|
- According to charles's law,
Text Solution
|
- The pressure of moist gas is higher than pressure of dry gas.
Text Solution
|
- Gases do not occupy volume and do not have force of attraction.
Text Solution
|
- The van der Waal equation of gas is (P + (n^(2)a)/(V^(2))) (V - nb)...
Text Solution
|
- Surface tension and surface energy have different dimensions.
Text Solution
|
- The plot of PV vs P at particular temperature is called isovbar.
Text Solution
|
- Give reasons for the following in one or two sentences. (a) A bottle...
Text Solution
|
- Can a gas with a = 0 be liquefied?
Text Solution
|
- The van der waals constants have same values for all the gases.
Text Solution
|
- All the molecules in a given sample of gas move with same speed.
Text Solution
|