A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Assertion-Reasoning)|24 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Integer)|10 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correcttype)|32 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|11 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|33 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-STATES OF MATTER-Exercises (Single Correct)
- At low pressures, van der Waals' equation is written as (P+(a)/(V^(2))...
Text Solution
|
- Ideal gas equation in terms of KE per unit volume, E, is
Text Solution
|
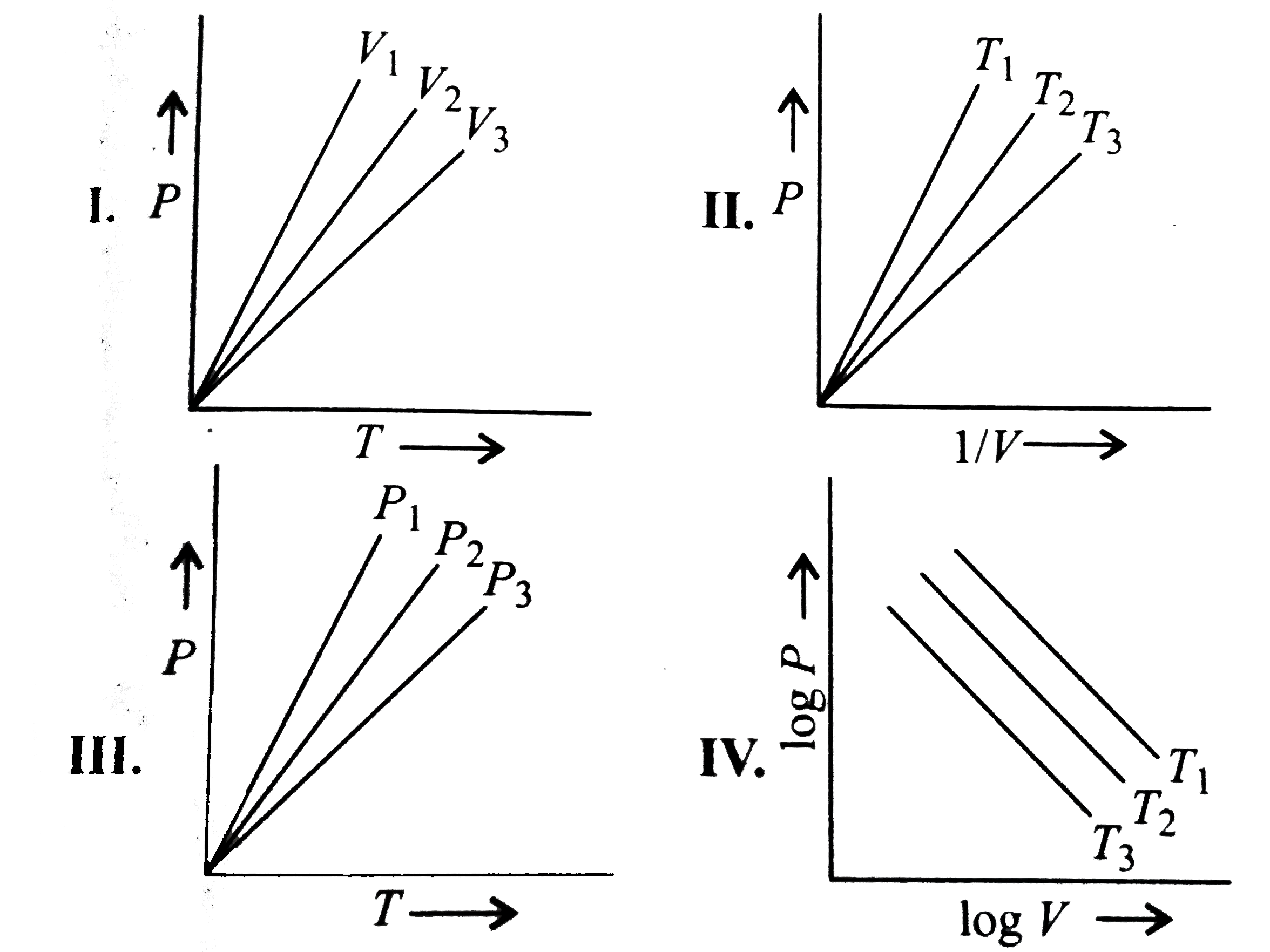
- For 1 mol of an ideal gas, V(1)gtV(2)gtV(3) in fig. (I),T(1)gtT(2)gtT(...
Text Solution
|
- I, II, and III are three istherms, respectively, at T(1), T(2), and T(...
Text Solution
|
- A quantity of hydrogen gas occupies a volume of 30.0 mL at a certain t...
Text Solution
|
- A gas in an open container is heated from 27^(@)C to 127^(@)C The frac...
Text Solution
|
- The density of neon gas will be highest at
Text Solution
|
- A mixture of SO(2) and O(2) in the molar ratio 16:1 is diffused throug...
Text Solution
|
- A graph is plotted between log V and log T for 2 mol of gas at constan...
Text Solution
|
- A gas obeys P(V-b)=RT. Which of the following are correct about this g...
Text Solution
|
- The pressure of a real gas is less than the pressure of an ideal gas b...
Text Solution
|
- O(2) gas at STP contained in a flask was replaced by SO(2) under same ...
Text Solution
|
- At what temperature will hydrogen molecules have the same KE as nitrog...
Text Solution
|
- Select the correct statements. (I) Greater is humidity, lesser will ...
Text Solution
|
- The temperature to which a gas must be cooled before it can be liquifi...
Text Solution
|
- Distribution of molecules with velocity is represented by the curve ...
Text Solution
|
- The volume of helium is 44.8 L at
Text Solution
|
- Which gas shows real behaviour?
Text Solution
|
- For the non-zero volume of the molecules, real gas equation for n mol ...
Text Solution
|
- Actual graph for the given parameters in (Q.25) will be
Text Solution
|