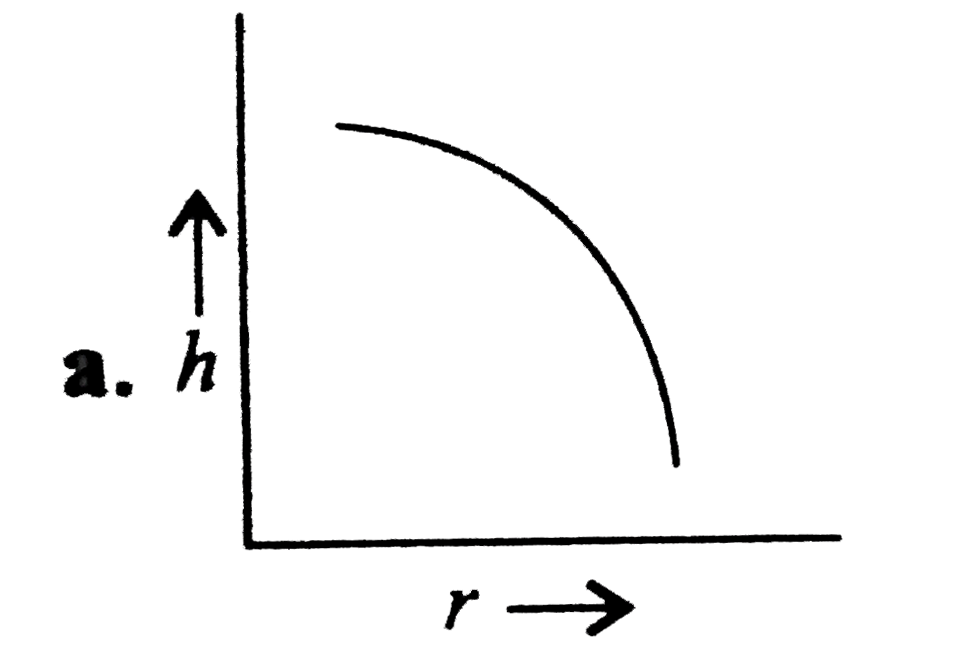
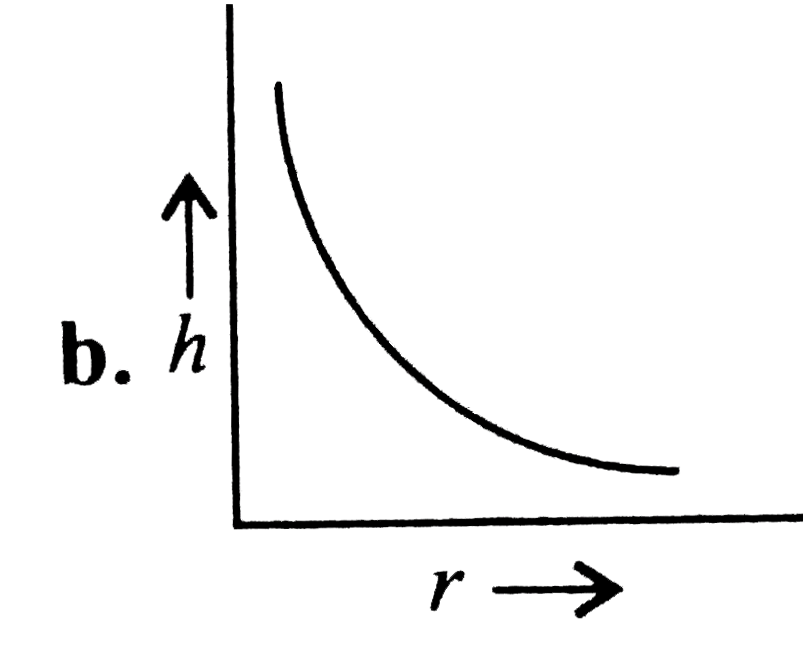
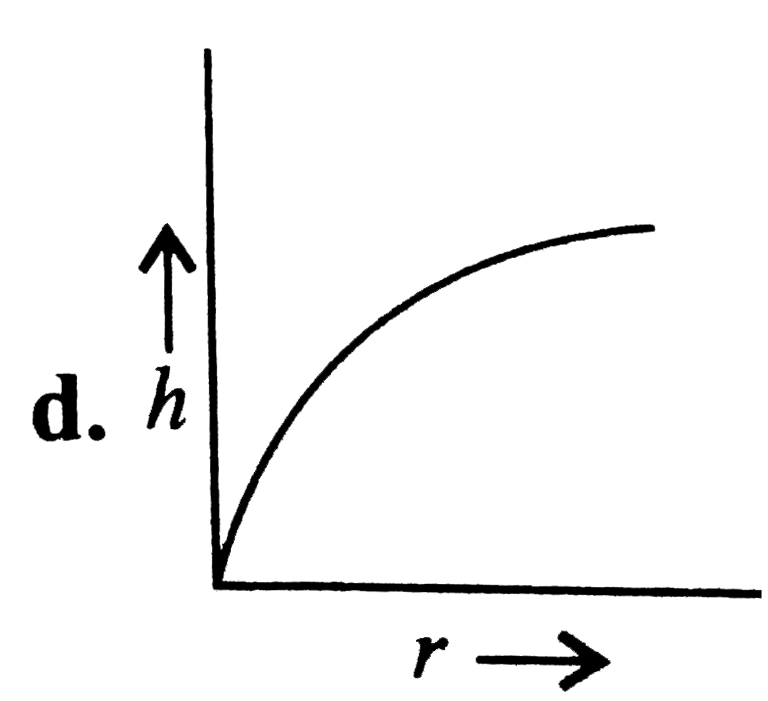
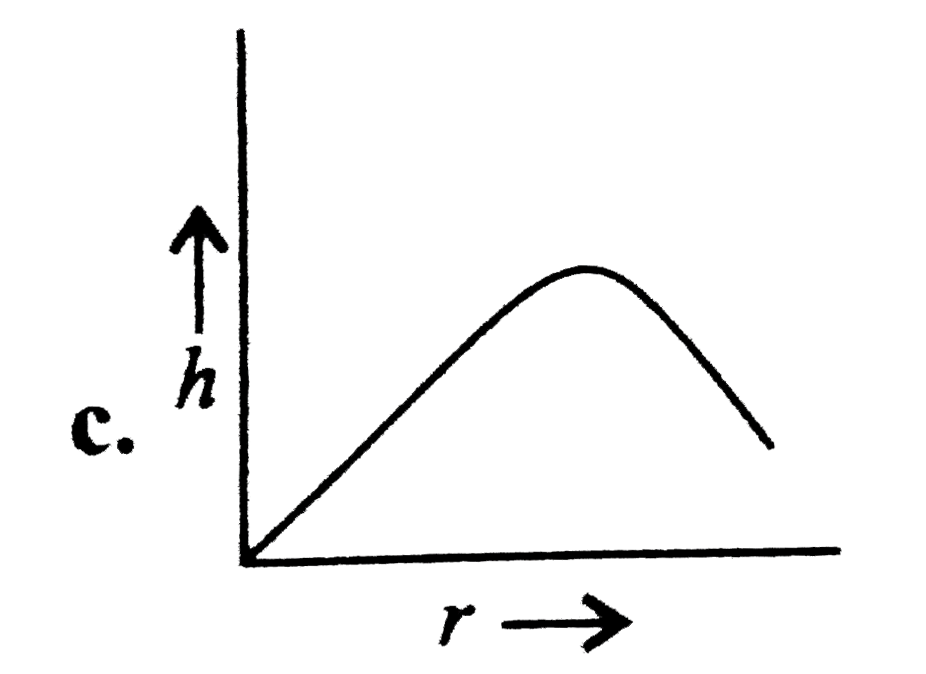A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Assertion-Reasoning)|24 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Integer)|10 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correcttype)|32 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|11 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|33 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-STATES OF MATTER-Exercises (Single Correct)
- It is eaiser to liquefy oxygen than hydrogen because.
Text Solution
|
- 2 mol 'H(2) is mixed with 2 gm of H(2). The molar heatr capacity at c...
Text Solution
|
- Which of the following graphs may represent the relation between the c...
Text Solution
|
- There is a depression in the surface of the liquid in a capillary when
Text Solution
|
- Surface tension does not very with
Text Solution
|
- Which among of the following has least surface tension?
Text Solution
|
- Units of coefficient of viscosity are
Text Solution
|
- The quantity (PV)/(k(B)T) represents the ( k(B):Boltzmann constant)
Text Solution
|
- 1.0 litre of N(2) and 7/8 litre of O(2) at the same temperature and pr...
Text Solution
|
- The value of PV for 5.6 L of an ideal gas is ……… RT at NTP.
Text Solution
|
- If a gas expands at contant temperature, it indicates that :
Text Solution
|
- The density of gas A is twice that to B at the same temperature. The m...
Text Solution
|
- Which of the following expression at constant pressure represents Char...
Text Solution
|
- A gas volume 100 cc is kept in a vessel at pressure 10.4 Pa maintained...
Text Solution
|
- A sample of gas occupies 100 mL at 27^(@)C and 740 mm pressure. When i...
Text Solution
|
- At 25^(@)C and 730 mm pressure, 730 mL of dry oxygen was collected. If...
Text Solution
|
- The density of a gas at 27^(@)C and 1 atm is d. Pressure remaining con...
Text Solution
|
- The kinetic theory of gases predicts that total kinetic energy of a ga...
Text Solution
|
- At STP, the order of mean square velocity of molecules of H(2), N(2), ...
Text Solution
|
- Which of the following statements is wrong for gases? (a)Gases do not...
Text Solution
|