A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
STATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Assertion-Reasoning)|24 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Integer)|10 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correcttype)|32 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|11 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|33 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-STATES OF MATTER-Exercises (Single Correct)
- Which of the following statements is wrong for gases? (a)Gases do not...
Text Solution
|
- 3.2 g oxygen is diffused in 10 min. In similar conditions, 2.8 g nitro...
Text Solution
|
- At what temperature will the molar kinetic energy of 0.3 mol of He be ...
Text Solution
|
- Which one of the following statements is not correct about the three s...
Text Solution
|
- Which of the following is true about gaseous state?
Text Solution
|
- Which of the following is not a correct postulate of kinetic theory of...
Text Solution
|
- In the van der Waals equation
Text Solution
|
- According to kinetic theory of gases, for a datomic molecule.
Text Solution
|
- A vessel is filled with a mixture of oxygen and nitrogen. At what rati...
Text Solution
|
- Select the correct statement. In the gas equation, PV=nRT
Text Solution
|
- When is the deviation more in the behaviour of a gas from the ideal ga...
Text Solution
|
- An ideal gas obeying the kinetic theory of gases can be liquefied if
Text Solution
|
- Which of the following expressions correctly represents the relationsh...
Text Solution
|
- Which expression gives average speed of gas molecules?
Text Solution
|
- Under similar conditions, which of the following gas will have same va...
Text Solution
|
- 15 L of gas at STP is subjected to four different conditions of temper...
Text Solution
|
- A gaseous mixture contains oxygen and nitrogen in the ratio 1 : 4 by w...
Text Solution
|
- Among the plots of P vs V given below, which one corresponds to Boyle'...
Text Solution
|
- The pressure of a gas is due to ........... exerted by its molecules p...
Text Solution
|
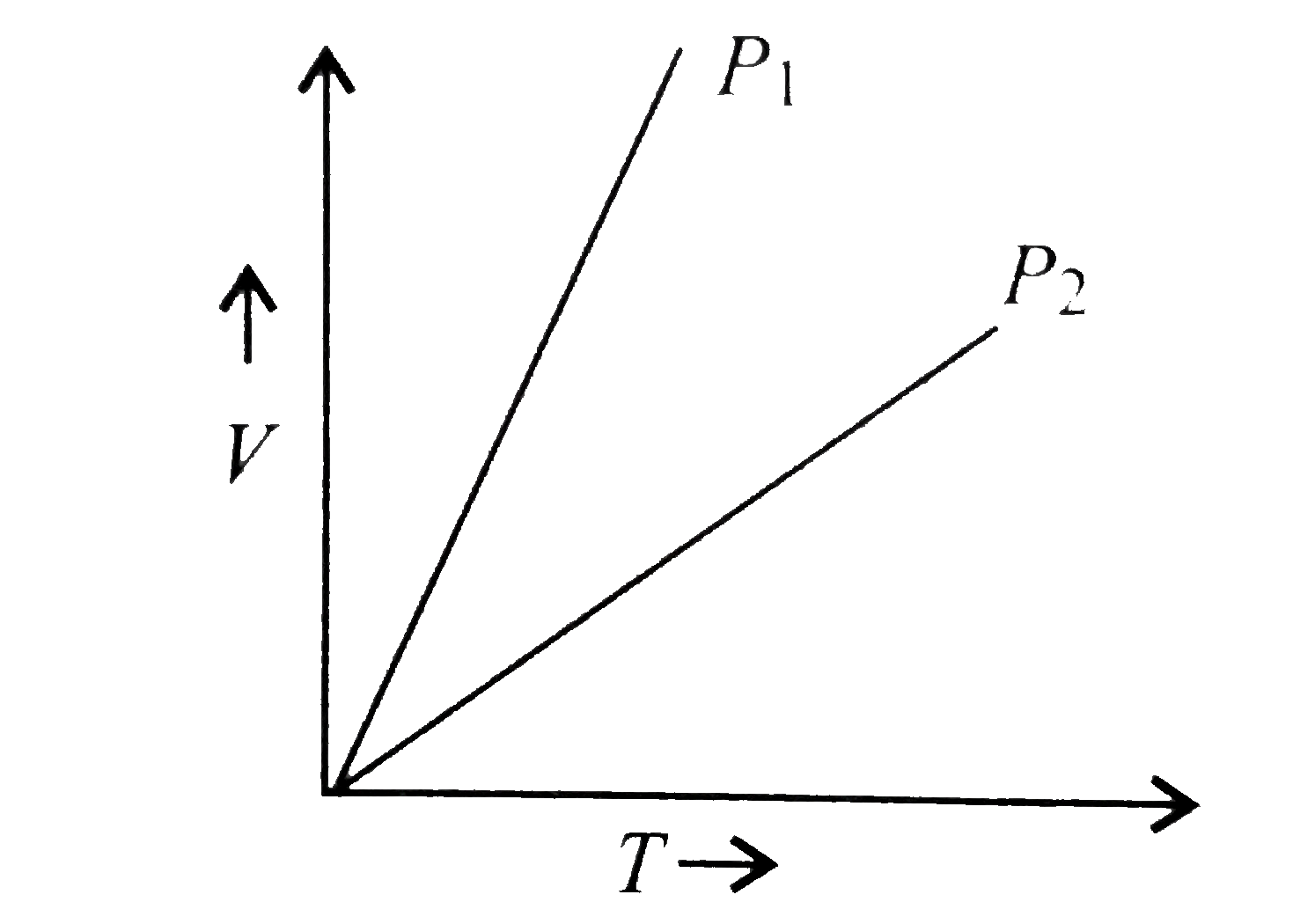
- V vs T curves at different pressures P(1) and P(2) for an ideal gas ar...
Text Solution
|