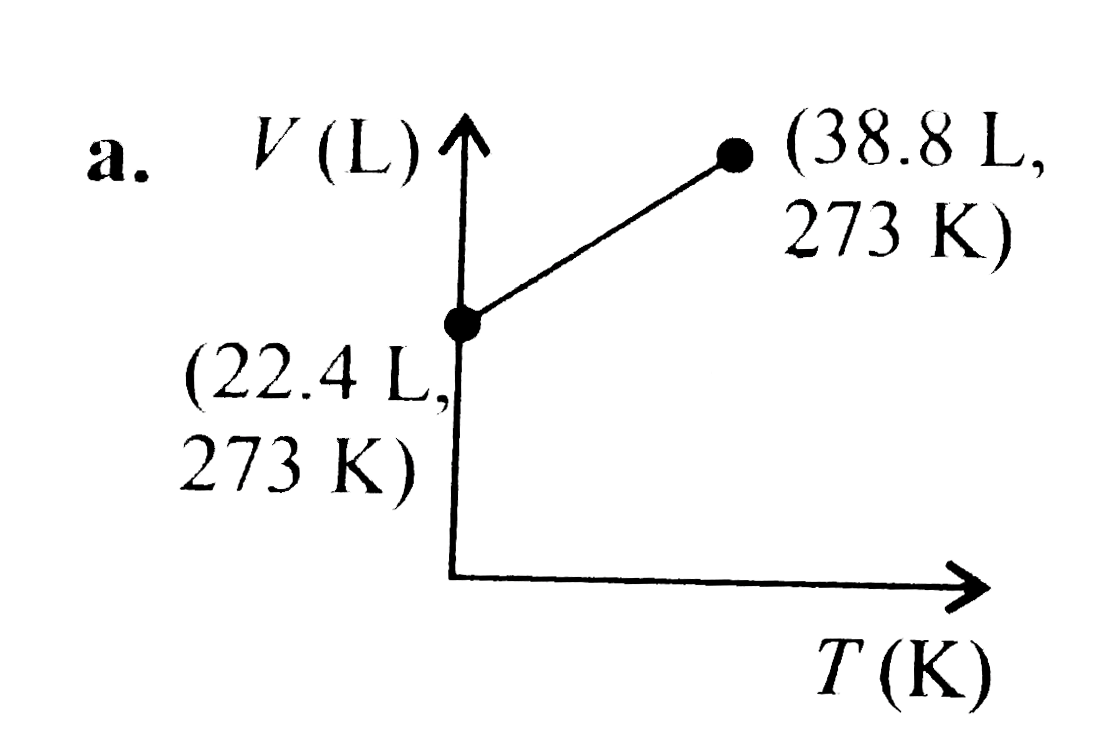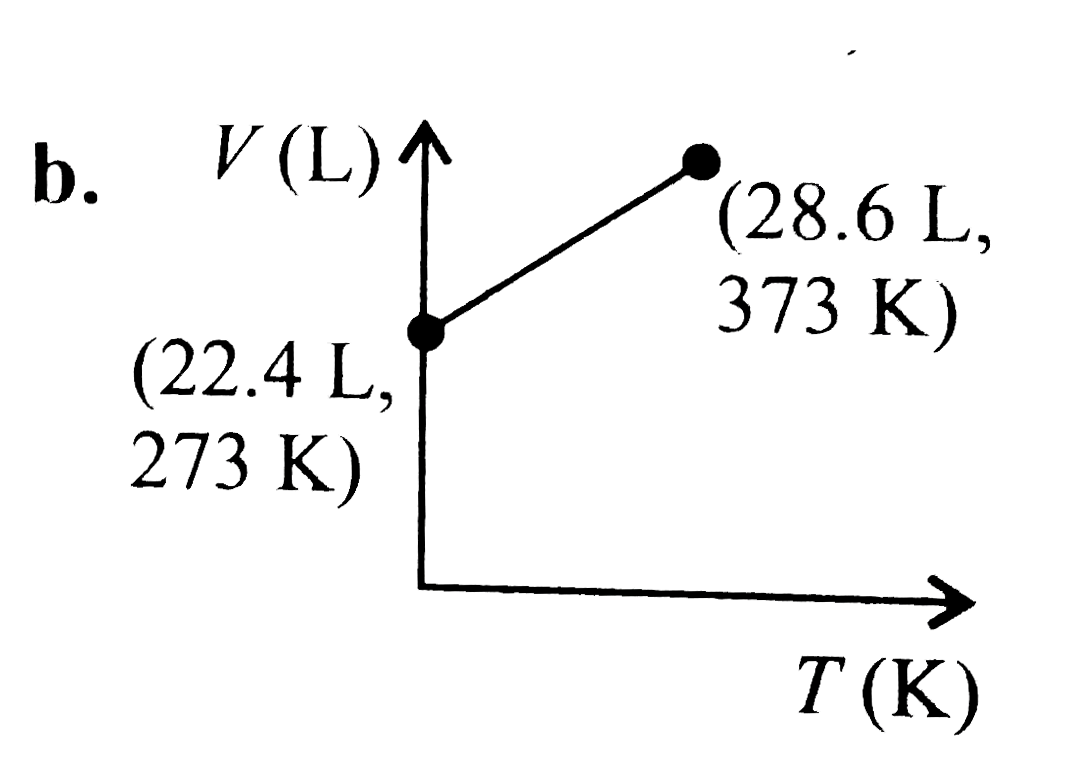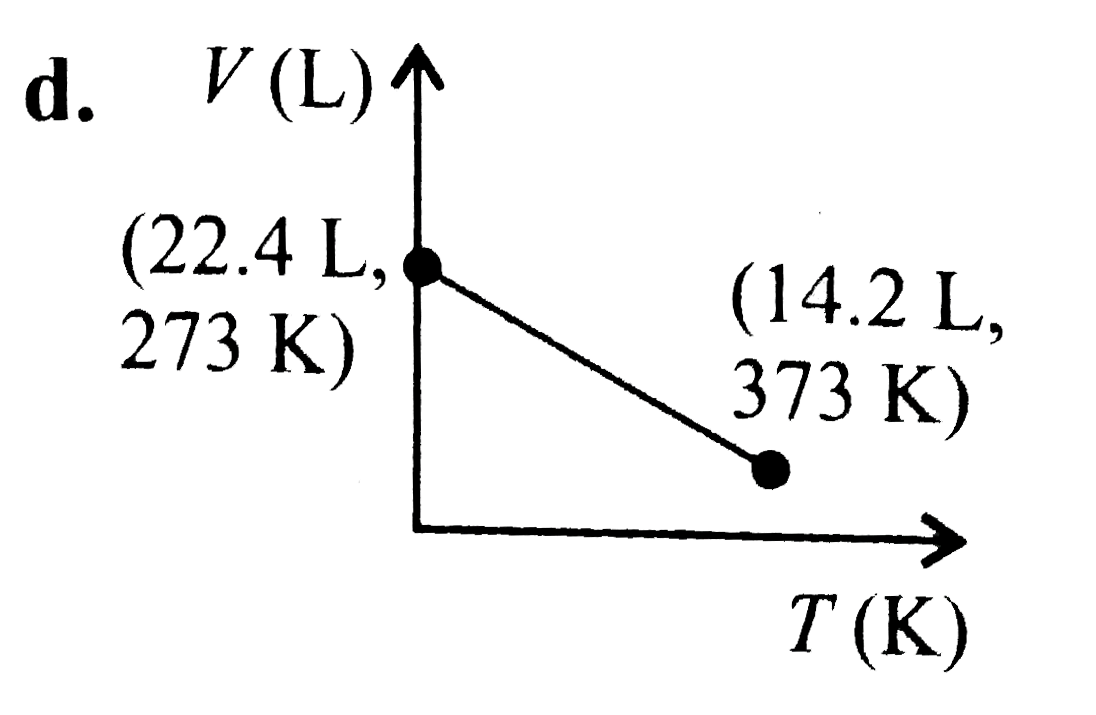A
B
C
D
Text Solution
AI Generated Solution
Topper's Solved these Questions
STATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Archives ( Assertion-Reasoning)|2 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Integer)|1 VideosSTATES OF MATTER
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Multiple Correct)|3 VideosSOME BASIC CONCEPTS AND MOLE CONCEPT
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|11 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|33 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-STATES OF MATTER-Archives (Single Correct)
- The density of neon gas will be highest at
Text Solution
|
- The rate of diffusion of methane is twice that of X. The molecular mas...
Text Solution
|
- Accoring to the kinetic theory of gases, for a diatomic molecule
Text Solution
|
- At constant volume, for a fixed number of moles of a gas, the pressure...
Text Solution
|
- Equal mass of methane and oxygen are mixed in an empty container at 2...
Text Solution
|
- The ratio between the root mean square velocity of H(2) at 50 K and th...
Text Solution
|
- X mL of H(2) gas effuses through a hole in a container in 5 seconds. T...
Text Solution
|
- The value of compressibility factor for an ideal gas is equal to 1.
Text Solution
|
- According to Graham's law, at a given temperature the ratio of diffusi...
Text Solution
|
- A gas will approach ideal behaviour at
Text Solution
|
- The RMS velocity of hydrogen is sqrt7 times the RMS velocity of nitrog...
Text Solution
|
- The compressibility of a gas is less than unity at STP. Therefore,
Text Solution
|
- At 100°C and 1 atm, if the density of liquid water is 1.0 g cm^(-3) an...
Text Solution
|
- The root mean square velocity of an ideal gas at constant pressure var...
Text Solution
|
- Which of the following volume-temperature (V-T) plots represents the b...
Text Solution
|
- If temperature increases, the surface tension of a liquid
Text Solution
|
- Positive deviation from ideal behaviour takes place because of
Text Solution
|
- For a monatomic gas, kinetic energy = E. The relation with rms velocit...
Text Solution
|
- The ratio of the rate of diffusion of helium and methane under indenti...
Text Solution
|
- The term that is correct for the attractive forces present in a real g...
Text Solution
|