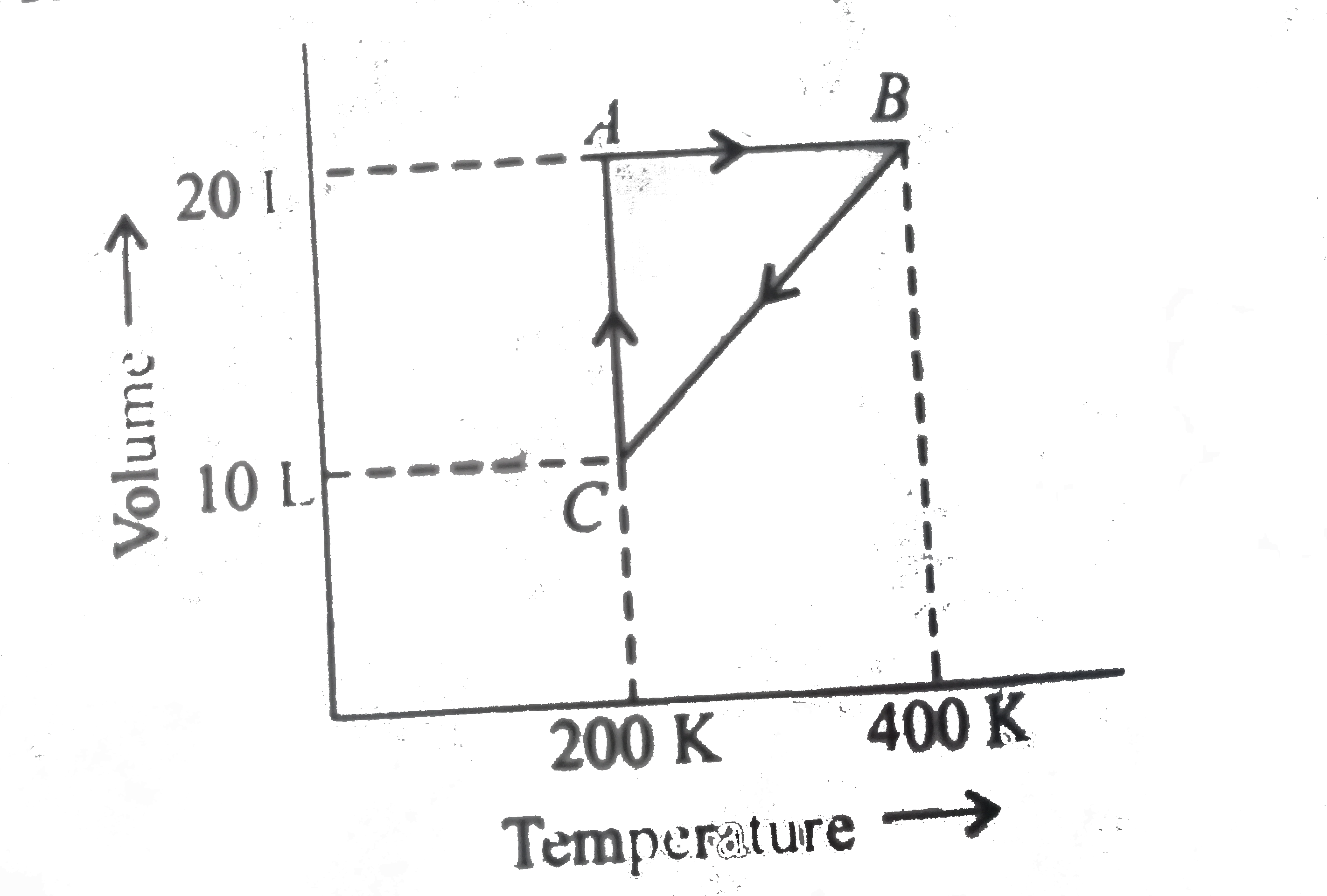
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct)|50 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple correct Answer Type|2 VideosTHERMODYNAMICS
CENGAGE CHEMISTRY ENGLISH|Exercise Paragraph for Problem|1 VideosSTOICHIOMETRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|33 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-THERMODYNAMICS-Exercises (Linked Comprehension)
- A change in the free energy of a system at constant temperature and pr...
Text Solution
|
- A change in the free energy of a system at constant temperature and pr...
Text Solution
|
- A change in the free energy of a system at constant temperature and pr...
Text Solution
|
- A change in the free energy of a system at constant temperature and pr...
Text Solution
|
- Identify the correct statement for change of Gibbs free energy for a s...
Text Solution
|
- Process A rarr B represents
Text Solution
|
- The pressure at C is
Text Solution
|
- Work done in the process C rarrA is
Text Solution
|
- The process which occurs in going from B rarr C is
Text Solution
|
- The pressures at A and B in the atmosphere are, respectively,
Text Solution
|
- The thermodynamic property that measures the extent of molecular disor...
Text Solution
|
- The thermodynamic property that measures the extent of molecular disor...
Text Solution
|
- The thermodynamic property that measures the extent of molecular disor...
Text Solution
|
- For which of the following cases, DeltaS = (DeltaH)/(T)?
Text Solution
|
- The thermodynamic property that measures the extent of molecular disor...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|
- The pressure-volume of varies thermodynamic process is shown in graphs...
Text Solution
|