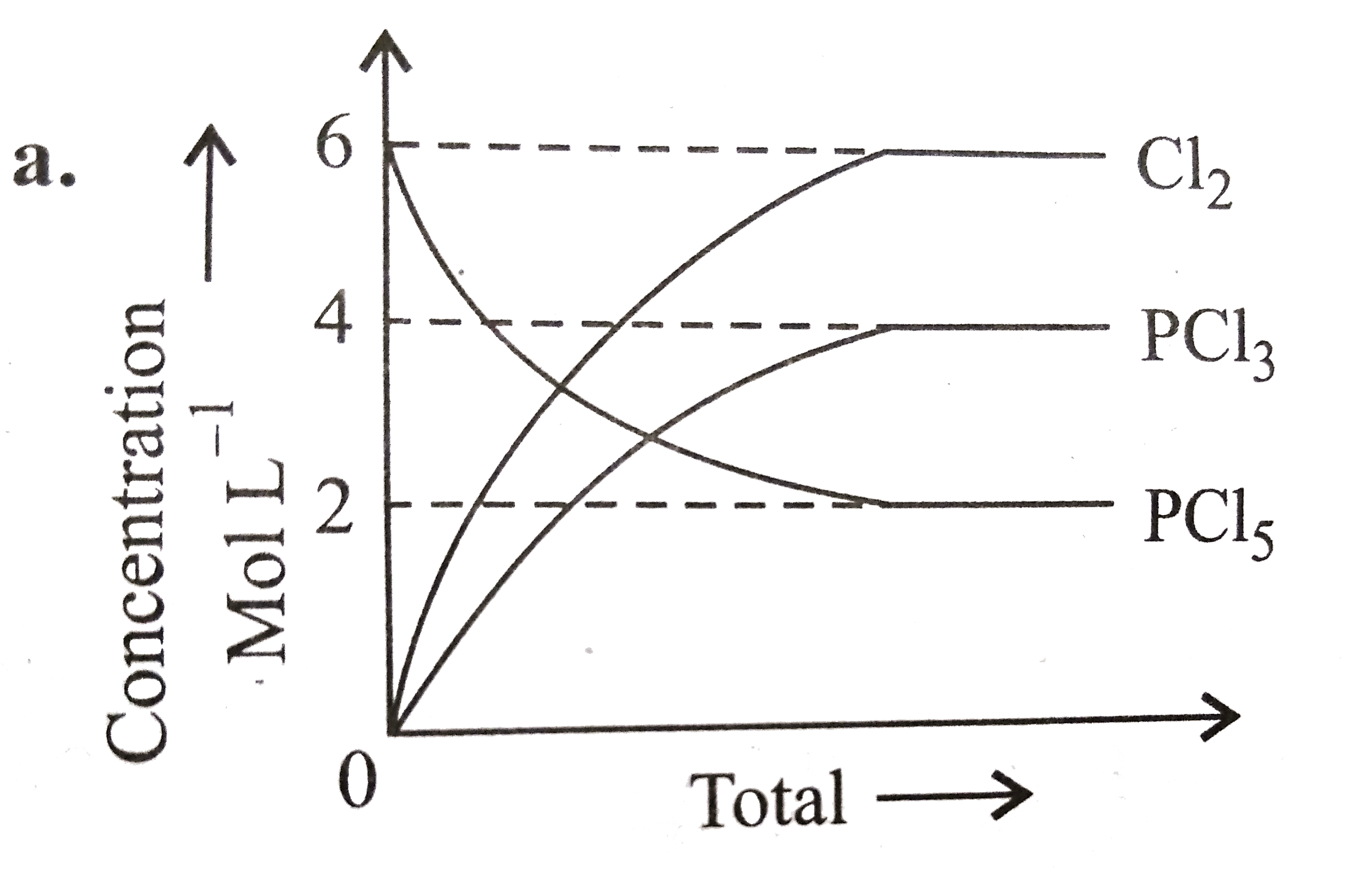
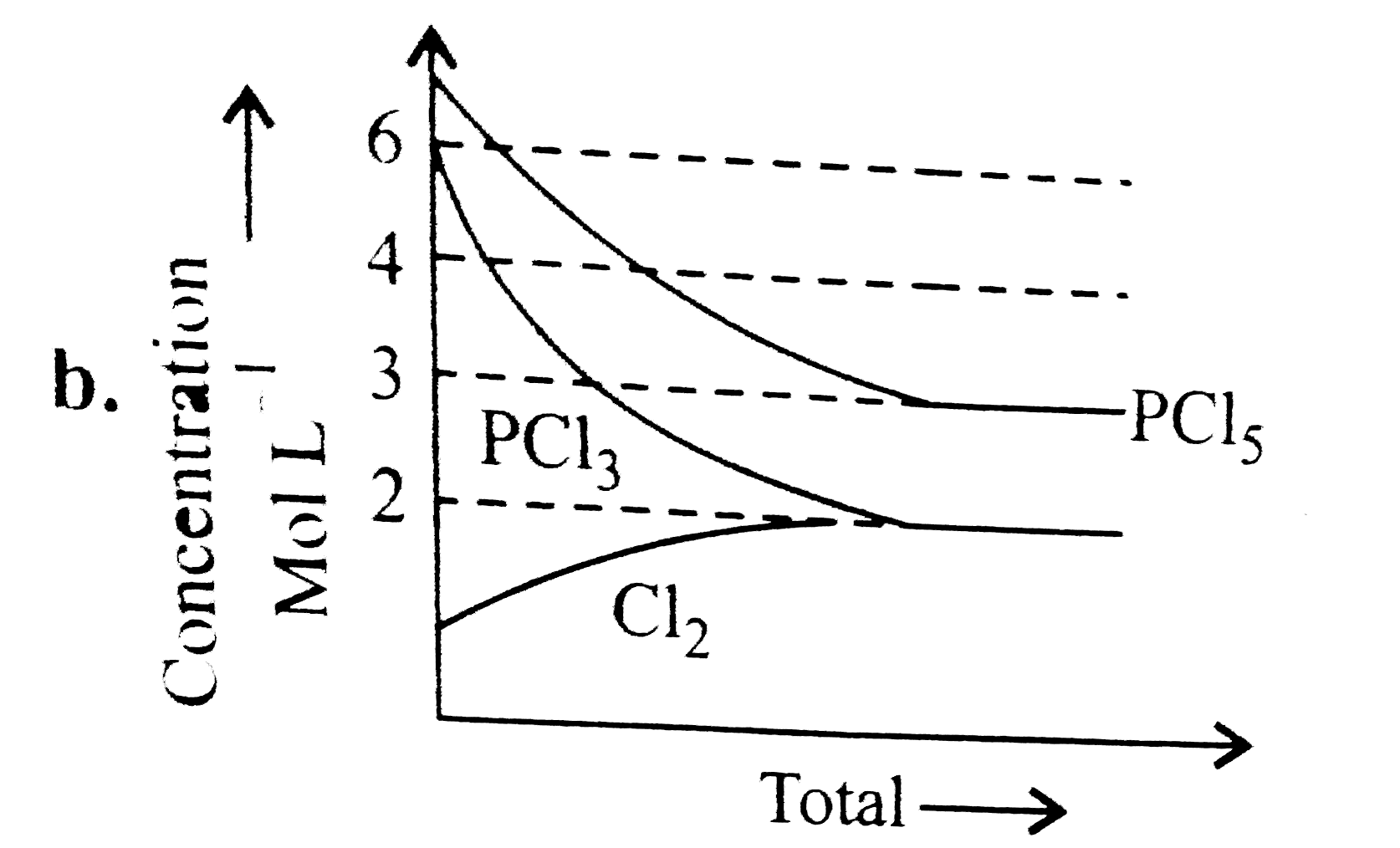
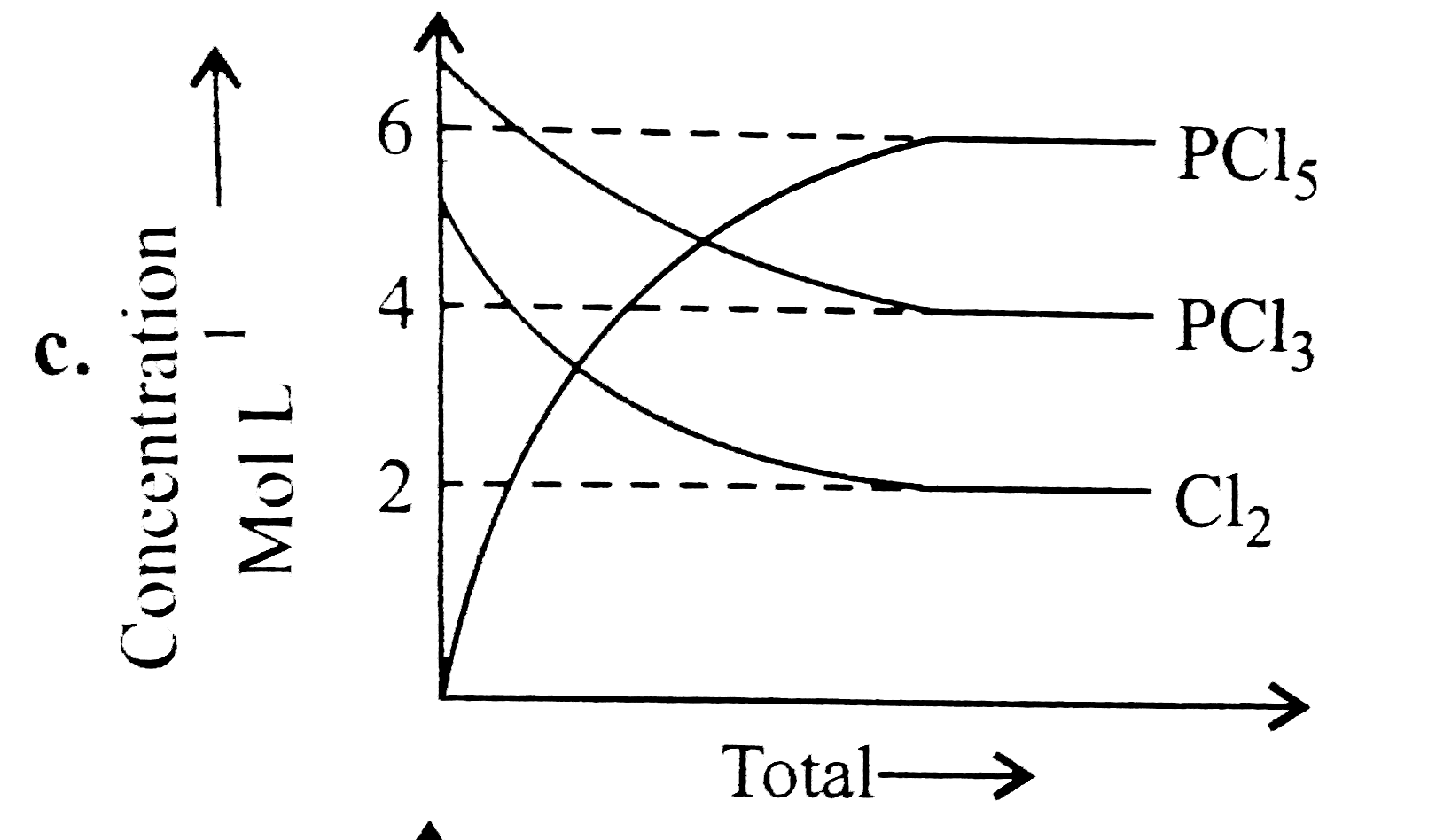
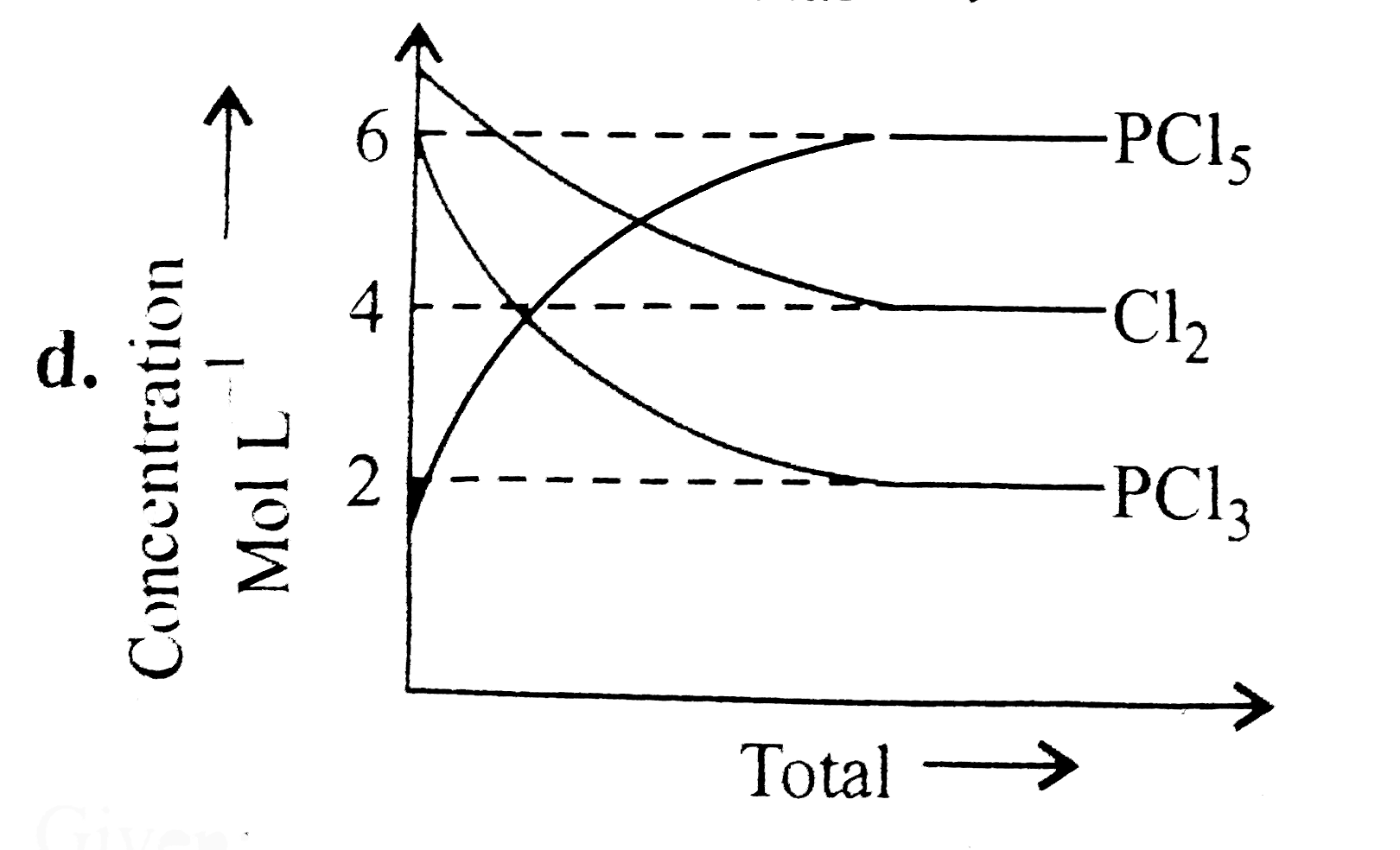
A
B
C
D
Text Solution
AI Generated Solution
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Applicationexercise 7.1|53 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 7.2|40 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|15 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive Type|3 Videos
Similar Questions
Explore conceptually related problems