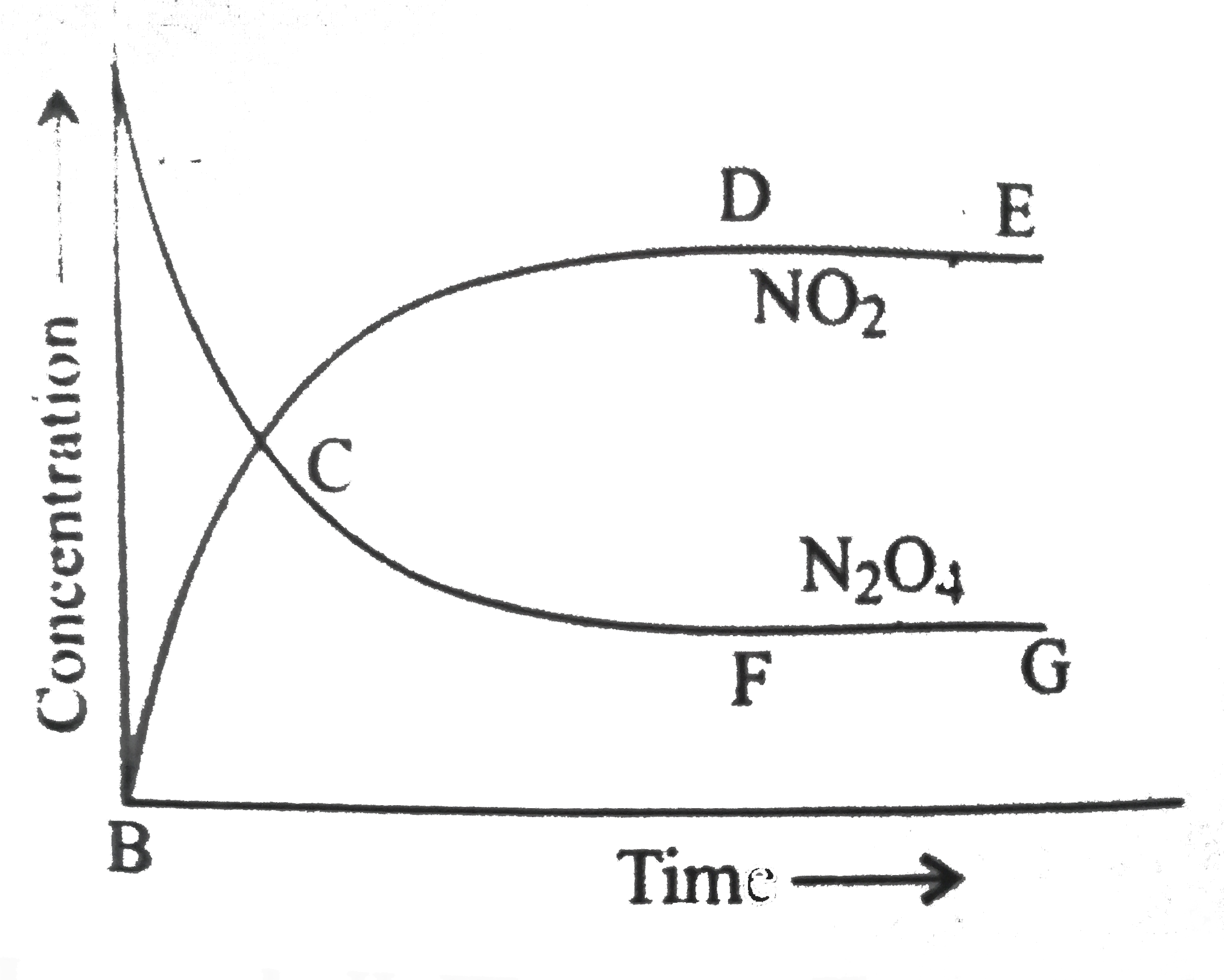
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Subjective)|46 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Linked Comprehensive)|54 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Applicationexercise 7.1|53 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|15 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive Type|3 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL EQUILIBRIUM-Ex 7.2
- In which of the following equilibrium ,change in volume of the system ...
Text Solution
|
- In the dissociation of 2HI hArr H(2)+I(2), the degree of dissociation ...
Text Solution
|
- In line kilns, the following reaction, CaCO(3)(s) hArr CaO(s)+CO(2)(...
Text Solution
|
- Which among the following reactions will be favoured at low pressure?
Text Solution
|
- If E(f) and E(r) are the activation energies of forward and backward r...
Text Solution
|
- K(p) for a reaction at 25^(@)C is 10 atm. The activation energy for fo...
Text Solution
|
- The concentration of a pure solid or liquid phase is not include in th...
Text Solution
|
- For an equilibrium reaction involving gases, the forward reaction is f...
Text Solution
|
- For the reaction, PCl(3)(g)+Cl(2)(g) hArr PCl(5)(g), the position of e...
Text Solution
|
- What are the favourable conditions for the synthesis of ammonia.
Text Solution
|
- Which of the following change will shift the reaction in forward direc...
Text Solution
|
- In a vessel containing SO(3), SO(2) and O(2) at equilibrium, some heli...
Text Solution
|
- Vapour density of the equilibrium mixture of NO(2) and N(2)O(4) is fou...
Text Solution
|
- Calculate the pressure of CO(2) gas at 700 K in the heterogenous equil...
Text Solution
|
- The equilibrium constant K(p(2)) and K(p(2)) for the reactions A hArr ...
Text Solution
|
- For I(2)(g) hArr 2I(g), K(p)=1.79xx10^(-10). The partial pressure of I...
Text Solution
|
- Calculate the volume percent of chlorine gas at equilibrium in the dis...
Text Solution
|
- N(2)O(4) hArr 2NO(2), K(c)=4. This reversible reaction is studied grap...
Text Solution
|
- The equilibrium: P(4)(g)+6Cl(2)(g) hArr 4PCl(3)(g) is attained by ...
Text Solution
|
- N(2)O(4)(g) is dissociated to an extent of 20% at equilibrium pressure...
Text Solution
|