A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 8.5|6 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Subjective (Weak Acid And Weak Bases)|15 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 8.3|38 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective Archive (Subjective)|3 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning Type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-IONIC EQUILIBRIUM-Ex 8.4
- A solution constains Zn^(2+) ions and Cu^(2+) ions each of 0.02M. If t...
Text Solution
|
- The following pH range where the indicator shows change in colour are ...
Text Solution
|
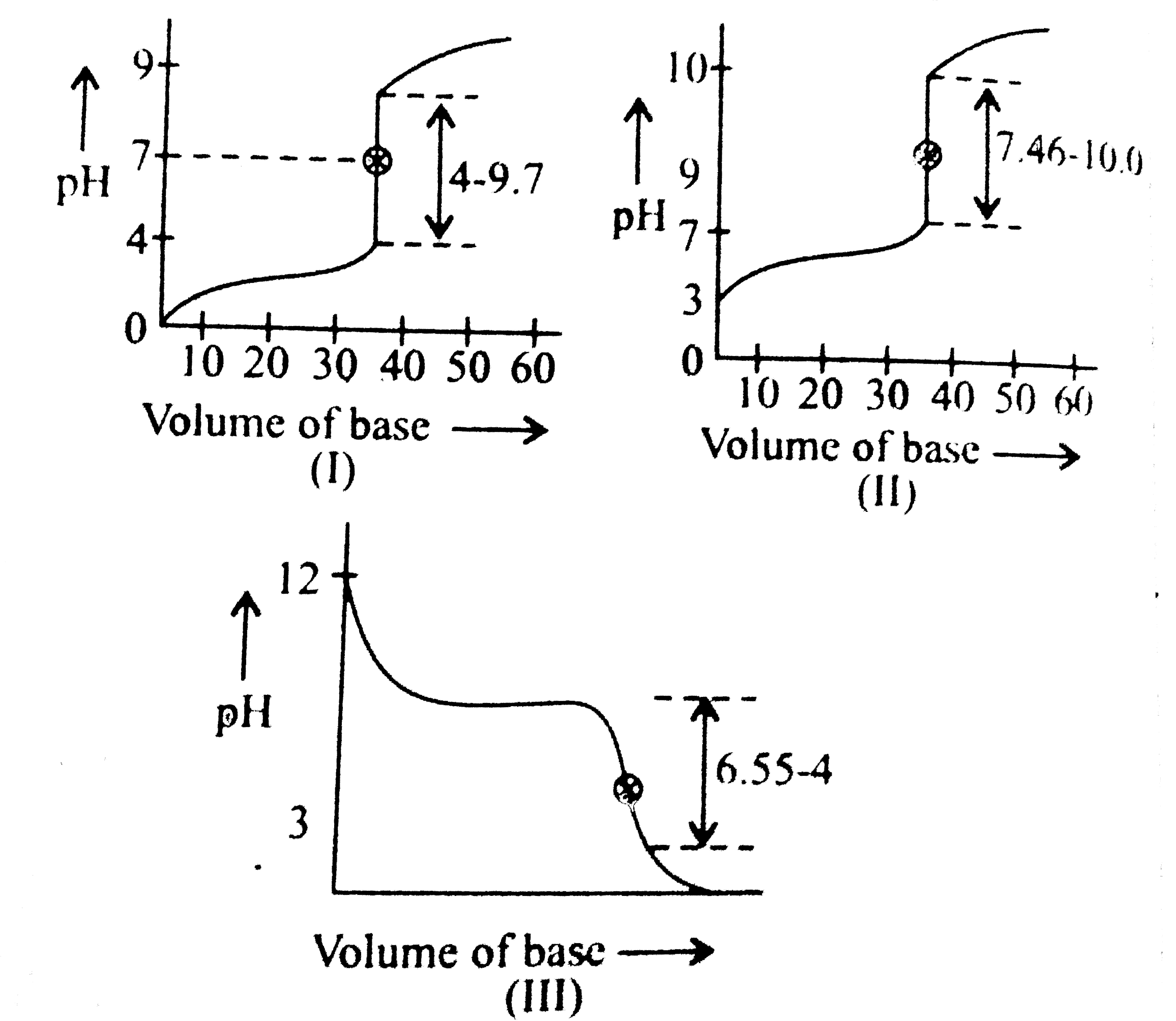
- The following acid base titration graphs are given: (I) Which of ...
Text Solution
|
- What indicators will be suitable for the following acid-base titration...
Text Solution
|
- A solution gives the following colours with different indicators: a....
Text Solution
|
- In the titration of NH(4)OH versus HCl, the pH of the solution at equi...
Text Solution
|
- The pH indicators are
Text Solution
|
- In which of the following acid-base titration, the pH is greater than ...
Text Solution
|
- Strong acids are generally used as standard solution in acid-base titr...
Text Solution
|
- The best indicator for detection of end point in titration of a weak a...
Text Solution
|
- The precipitate of CaF(2) (K(sp)=1.7xx10^(-10)) is obtained when equal...
Text Solution
|
- The solubility of A(2)B(3) is "x mol dm"^(-3). Its K(sp) is
Text Solution
|
- The pH of Ca(OH)(2) is 10.6 at 25^(@)C. K(sp) of Ca(OH)(2) is
Text Solution
|
- Solubility of AgI in 0.05M BaI(2) solution is 10^(-15)M. The solubilit...
Text Solution
|
- Solubility of a solute in water is dependent on temperature as given b...
Text Solution
|
- The solubility of CaF(2) in a solution of 0.1M Ca(NO(3))(2) is
Text Solution
|
- The volume of water needed to dissolve 1mg of PbSO(4) (K(sp) = 1.44 xx...
Text Solution
|
- The volume of water needed to prepare a satured solution of Ag^(o+) ha...
Text Solution
|
- How many grams of KBr can be added to 1L of 0.12 M solution of AgNO(3)...
Text Solution
|
- The solubility of silver benzoate (C(6)H(5)COOAg) in H(2)O and in a bu...
Text Solution
|