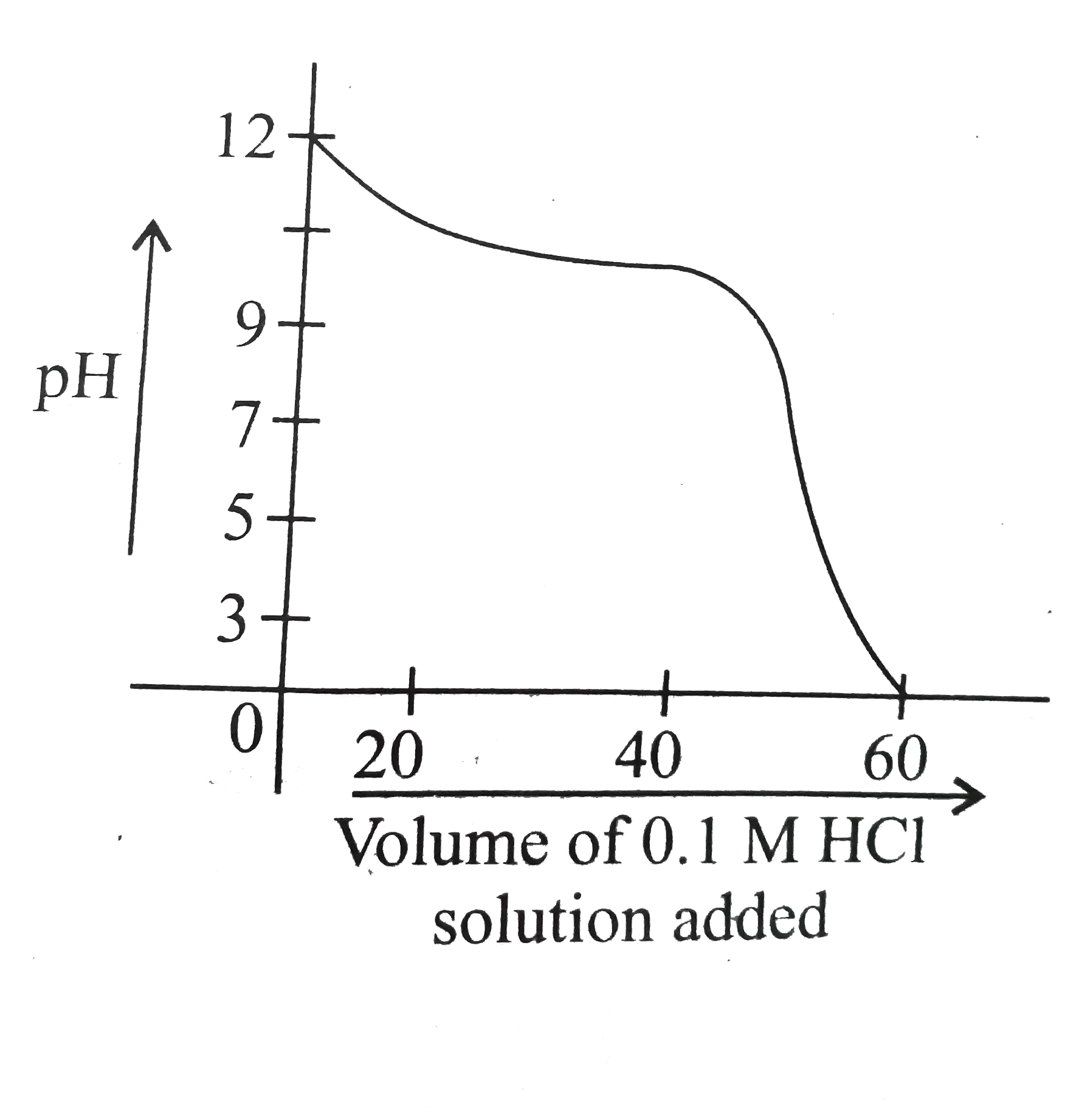
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
IONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|121 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Reasoning|36 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|45 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective Archive (Subjective)|3 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning Type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-IONIC EQUILIBRIUM-Exercises Multiple Correct
- 0.1mol of RNH(2)(K(b) = 5 xx 10^(-4)) is mixed with 0.08mol of HC1 and...
Text Solution
|
- When weak base solution (50mL of 0.1 N NH(4)OH) is titrated with stron...
Text Solution
|
- Which of the following statements about a weak acid strong base titrat...
Text Solution
|
- An acid-base indicator has K(a) = 10^(-5). The acid form of the indica...
Text Solution
|
- When HCI gas is passed through a saturated solution of common salt, pu...
Text Solution
|
- Excess of Ag(2)SO(4)(s), BaSO(4)(s), and Ba(3)(PO(4))(2)(s) are simult...
Text Solution
|
- A solution is found to contain [CI^(Theta)] = 1.5 xx 10^(-1)M, [Br^(Th...
Text Solution
|
- HgCrO(4) just begins to peripitate when equal volumes of 4 xx 10^(-4)M...
Text Solution
|
- What is general criteria of choosing a suitable indicator for a given ...
Text Solution
|
- Which of the following are true for an acid- base titration?
Text Solution
|
- An acid-base indicator has K(a) = 3.0 xx 10^(-5). The acid form of the...
Text Solution
|
- At the end point, there is a sharp change of colour in the indicator. ...
Text Solution
|
- For a series of indicators, the colour and pH range over which colour ...
Text Solution
|
- H(3)PO(4)hArr H^(o+) +H(2)PO(4)^(Theta), K(a(1)): H(2)PO(4)^(Theta) ...
Text Solution
|
- Aqueous solution of HNO(3),CH(3),CH(3)COOH, and CH(3)COOK of identical...
Text Solution
|
- To which of the solution, addition of water would not effect the pH ? ...
Text Solution
|
- Which of the following salt solutions has pH lt7 ? .
Text Solution
|
- Which of the following represents hydrolysis ? .
Text Solution
|
- The pH values of aqueous solutions of which of the following compounds...
Text Solution
|
- In H(3)PO(4) which of the following is true?
Text Solution
|