Text Solution
Verified by Experts
Topper's Solved these Questions
NCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Thermodynamics|50 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Chemical Equilibrium|73 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Redox Reaction|25 VideosISOMERISM
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning Type|1 VideosORGANIC REACTION MECHANISM
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive|6 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-NCERT BASED EXERCISE-Atomic Structure
- What will be the pressure of the gaseous mixture when 0.5 L of H(2) at...
Text Solution
|
- Density of a gas is found to be 5.46 g//dm^(3) at 27 .^(@)C and 2 bar ...
Text Solution
|
- 34 . 05 mL of phosphorus vapours weigh 0.0625 g at 546 .^(@)C and 0.1 ...
Text Solution
|
- A student forgot to add the reaction mixture to the round bottomed fla...
Text Solution
|
- Calculate the temperature of 4.0 mol of a gas occupying 5 dm^3 at 3.32...
Text Solution
|
- calculte the total number of electrons present 1.4 g of dinitrogen gas...
Text Solution
|
- how much time would it take to distribute on Avogadro number of wheat ...
Text Solution
|
- Calculate the total pressure in a mixture of 8 g of dioxygen and 4 g o...
Text Solution
|
- Pay load is defined as the difference between the mass of displaced ai...
Text Solution
|
- Calculate the volume occupied by 8.8 g of CO(2) at 31.1^(@)C and 1 bar...
Text Solution
|
- 2.9 g of a gas at 95^(@)C occupied the same volume as 0.184 g of dihyd...
Text Solution
|
- A mixture of dihydrogen and dioxygen at one bar pressure contains 20% ...
Text Solution
|
- What would be the SI unit for the quantity PV^(2)T^(2)//n?
Text Solution
|
- In terms of Charles’ law explain why –273 ^(@)Cis the lowest possible ...
Text Solution
|
- Critical temperature for carbon dioxide and methane are 31.1 ""^(@)C ...
Text Solution
|
- Explain the physical significance of van der Waals parameters.
Text Solution
|
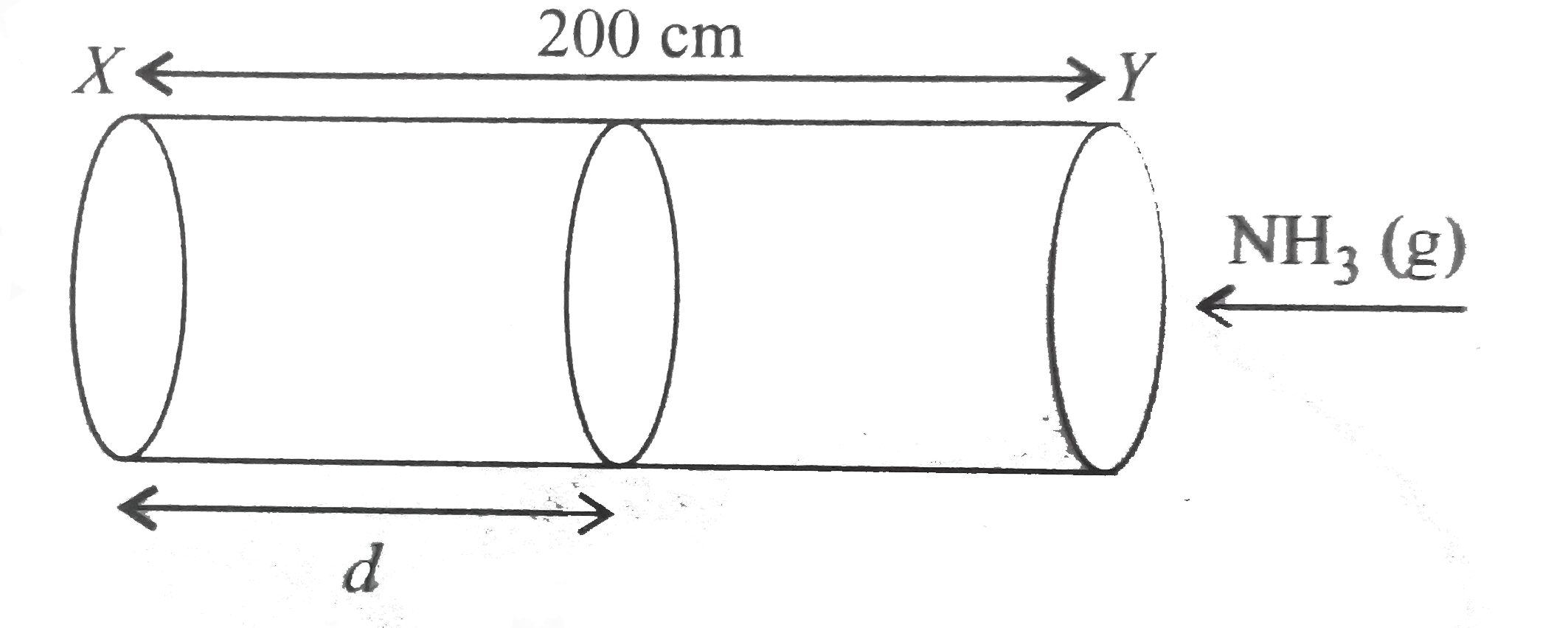
- A straight glass tube has two inlets X and Y at the two ends. The leng...
Text Solution
|
- From two identical holes, nitrogen and an unknown gas are leaked into...
Text Solution
|
- Equal volumes of two gases A and B diffuse through a porous pot in 20 ...
Text Solution
|
- Calculate the total and average kinetic energy of 32 g methane molecu...
Text Solution
|