A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Assertion-Reasoning)|19 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Interger)|9 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct)|39 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise SUBJECTIVE TYPE|4 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 2.3 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-SOLID STATE-Exercises (Single Correct)
- In the CaF(2) structure, the C.N of cations and anions are respectivel...
Text Solution
|
- A metallic crystal cystallizes into a lattice containing a sequence of...
Text Solution
|
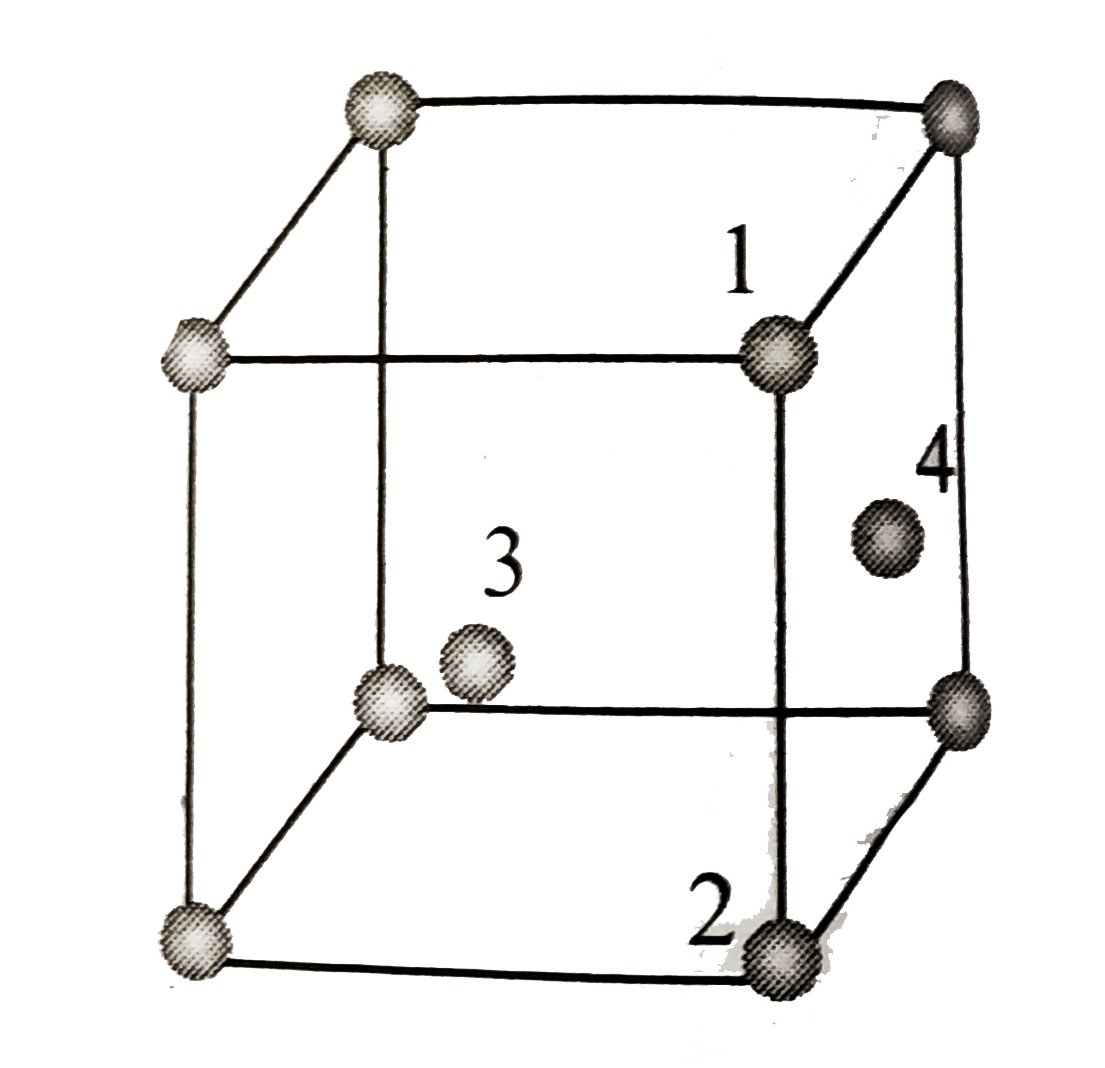
- In fcc unit cell, atoms are numbered as shown below. The atoms not t...
Text Solution
|
- The number of nearest neighbours and next nearest neighbours of an Na^...
Text Solution
|
- In the closet packing of atoms:
Text Solution
|
- The following diagram shows the arrangement of lattice points with a =...
Text Solution
|
- The number of atoms in 100 g of an fcc crystal with density = 10.0g cm...
Text Solution
|
- What is the effect of Frenkel defect on the density of ionic solids ?
Text Solution
|
- In a tetragonal crystal
Text Solution
|
- Which of the following is not a ferroelectric compound?
Text Solution
|
- The material used in solar cells contains
Text Solution
|
- If the lattice parameter of Si = 5.43 Å and the mass of Si atom is 28....
Text Solution
|
- The lacttice parameter of GaAs (radius of Ga = 1.22 Å, As = 1.25 Å) is
Text Solution
|
- In cubic ZnS (II-VI) compounds, if the radii of Zn and S atoms are 0.7...
Text Solution
|
- Na and Mg crystallize in bcc- and fcc-type crystals, the ratio of numb...
Text Solution
|
- In a close packed structure of mixed oxides , the lattice is composed ...
Text Solution
|
- An ionic solid A^(o+)B^(Θ) crystallizes as an bcc structure. The dist...
Text Solution
|
- An ionic solid A^(o+)B^(Θ) crystallizes as an fcc structure. If the e...
Text Solution
|
- The gamma-form of iron has fcc structure (edge length 386 pm) and beta...
Text Solution
|
- The density of an ionic compounds (Mw = 58.5) is 2.165 kg m^(-3) and ...
Text Solution
|