Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Fill In The Blanks)|15 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (True/False)|19 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Assertion-Reasoning)|19 VideosREDUCTION AND OXIDATION REACTION OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise SUBJECTIVE TYPE|4 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 2.3 (Objective)|9 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-SOLID STATE-Exercises (Interger)
- If a solid A^(o+)B^(ɵ) having ZnS Structure is heated so that the ions...
Text Solution
|
- Metal M of radius 50 nm is crystallized in fcc type and made cubical c...
Text Solution
|
- O(2-) ions are arranged in ccp in spinel strructure. A^(2+) ions occup...
Text Solution
|
- Find the coordination of Na^(o+) in Na(2)O.
Text Solution
|
- A body centered cubic lattice is made up of hollow sphere of B. ...
Text Solution
|
- In the figure given below, four parallelograms are shown. How many par...
Text Solution
|
- Cesium atoms are the largest neturally occurring atoms. The radius of ...
Text Solution
|
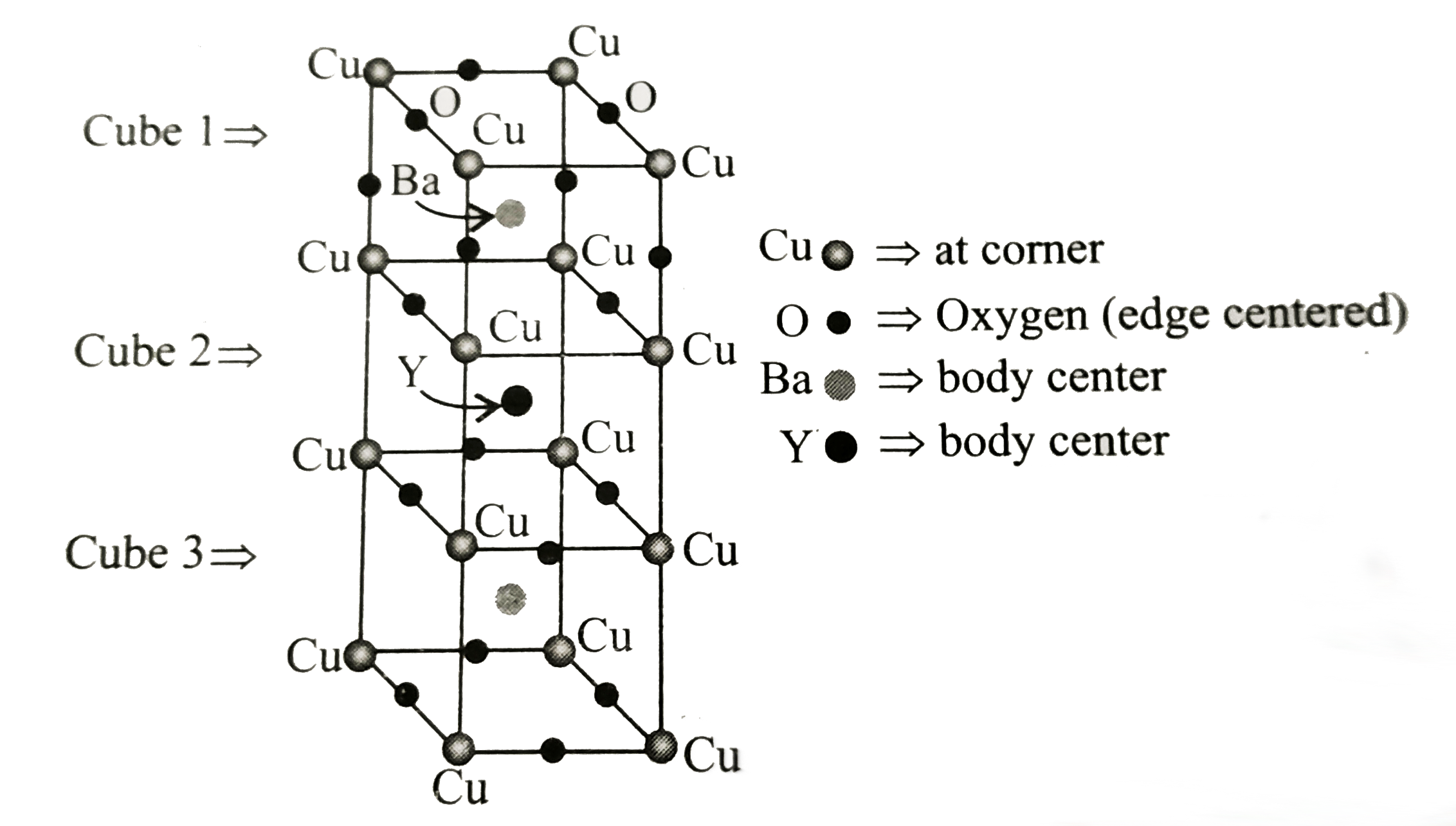
- The following figure shows the unit cell of a compound, i.e., a mixed ...
Text Solution
|
- A solid has a structure in which X atoms are located at cubic corners ...
Text Solution
|