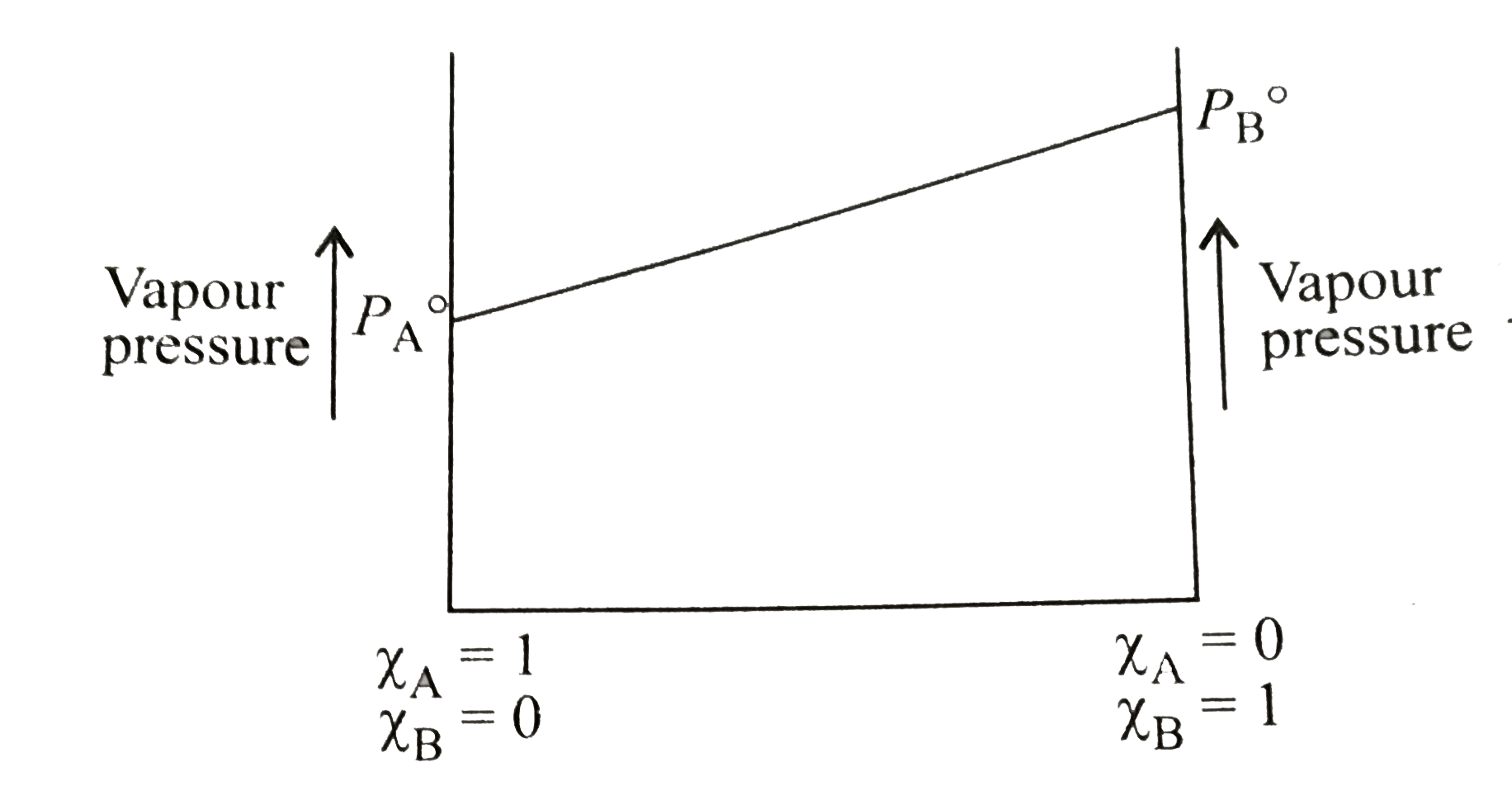
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correct)|99 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Assertion-Reasoning)|18 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Linked Comprehension)|58 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.2 (Objective)|9 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|2 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-SOLUTIONS-Exercises (Multiple Correct)
- Which of the following statements is/are correct?
Text Solution
|
- For a non-volatile solute
Text Solution
|
- To 10 mL of 1 M BaCl(2) solution 5mL of 0.5 M K(2)SO(4) is added. BaS...
Text Solution
|
- A difference between diffusion and osmosis is
Text Solution
|
- 1 mol benzene (P^(@)("benzene")=42 mm) and 2 mol toluence (P^(@)("to...
Text Solution
|
- Which of the following statements is/are correct?
Text Solution
|
- Consider the two solutions: I: 0.5 M NaCl aqueous solution at 25^(@)...
Text Solution
|
- Which pair(s) of liquids on mixing is/are expected to show no net volu...
Text Solution
|
- The following is a graph plotted between the vapour pressure of two vo...
Text Solution
|
- Consider the following solutions: I.1 M sucrose , II. 1 M KCl III...
Text Solution
|
- The osmotic pressure of a solution depends on
Text Solution
|
- 1.2575 g sample of [Cr(NH(3))(6)]SO(4)Cl(Mw=251.5) is dissolved to pre...
Text Solution
|
- 2 L of 1 molal solution of a complex salt CrCl(3).6H(2)O (Mw=266.5) s...
Text Solution
|
- Which of the following combinations are correct for a binary solution,...
Text Solution
|
- Effect of adding a non-volatie solute to a solvent is"
Text Solution
|
- Which of the following forms is an ideal solution?
Text Solution
|
- For a given value of degree of dissociation, which of the following ha...
Text Solution
|
- Choose the correct option:
Text Solution
|
- When acetone and chloroform are mixed, hydrogen bonding takes place be...
Text Solution
|
- A maxima or minima is obtained in the temperature composition curve of...
Text Solution
|