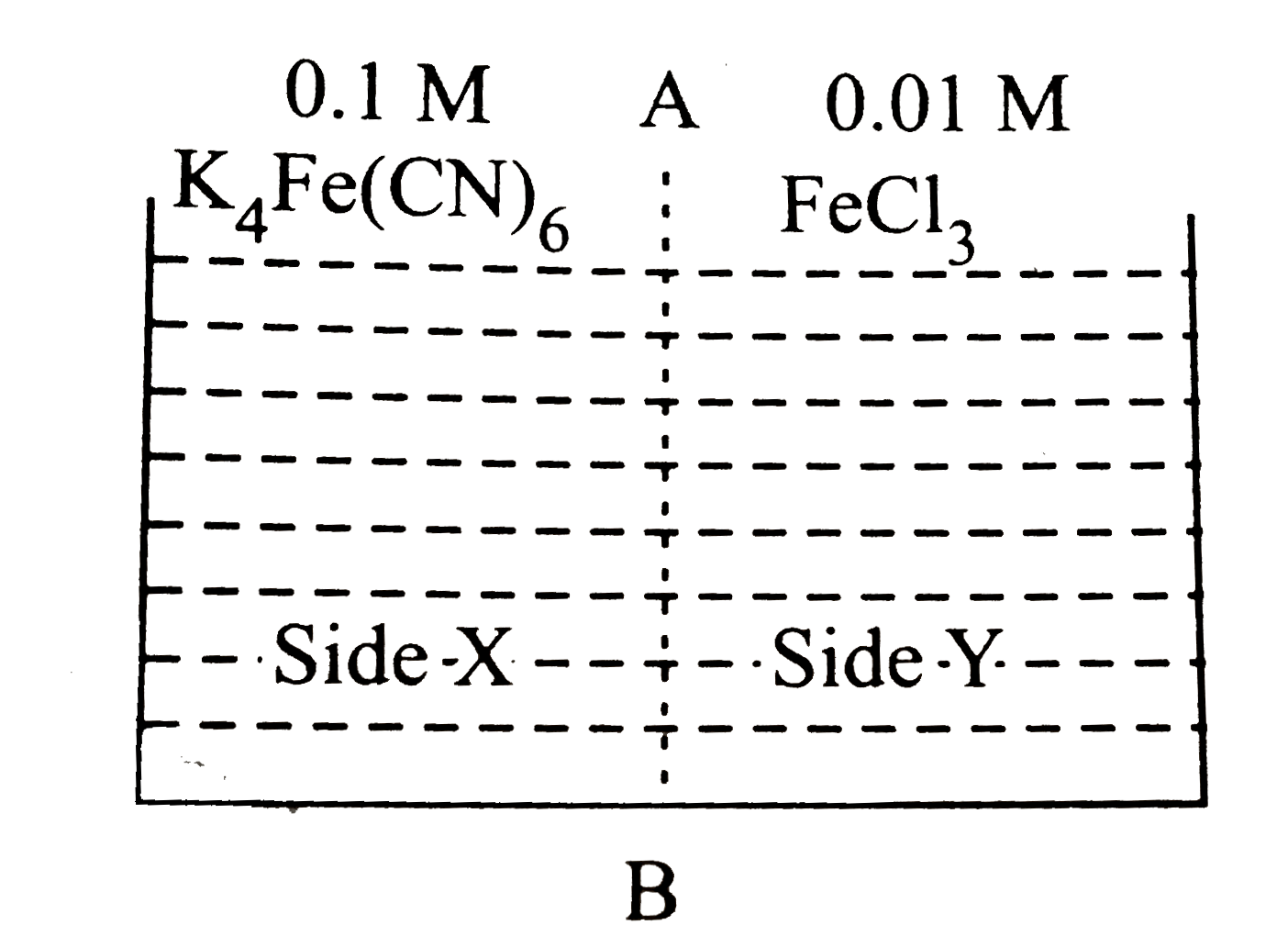A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Assertion-Reasoning)|18 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Interger)|8 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Multiple Correct)|25 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.2 (Objective)|9 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|2 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-SOLUTIONS-Exercises (Single Correct)
- Total Vapour pressure of mixture of 1molA(p(A)^(0)=150 "torr")and 2mol...
Text Solution
|
- The vapour pressure of pure benzene C(6)H(6) at 50^(@)C is 268 "torr"....
Text Solution
|
- Osmotic pressure of blood is 7.40 atm, at 27^(@)C. Number of moles of ...
Text Solution
|
- PtCl(4).6H(2)O can exist as a hydrated complex. 1 m aqueous solution h...
Text Solution
|
- For 1 molal solution of each compound maximum freezing point will be ...
Text Solution
|
- The depression in freezing point of 0.01 m aqueous CH(3)CooH solution ...
Text Solution
|
- pH of 0.1 M monobasic acid is found to be 2 . Hence its osmotic pressu...
Text Solution
|
- The lowering of vapour pressure due to a solute in a 1 m aqueous solut...
Text Solution
|
- The most likely of the following mixtures to be an ideal solution is
Text Solution
|
- Mole fraction of component A in vapour phase is chi(1) and that of com...
Text Solution
|
- Which has the maximum osmotic pressure at temperature T?
Text Solution
|
- The relative decreases in the vapour pressure of an aqueous solution c...
Text Solution
|
- Mixture of volatile components A and B has a total vapour pressure (in...
Text Solution
|
- FeCl(3) on reaction with K(4)[Fe(CN)(6)] in aqueous solution gives blu...
Text Solution
|
- 12.2 g of benzoic acid (Mw=122) in 100 g benzene has depression in fre...
Text Solution
|
- 25 mL of an aqueous solution of KCl was found to requires 20 mL of 1M ...
Text Solution
|
- Based on the given diagram, which of the following statements regardin...
Text Solution
|
- Following questions are based on the following activites (A) with obse...
Text Solution
|
- Following questions are based on the following activites (A) with obse...
Text Solution
|
- Following questions are based on the following activites (A) with obse...
Text Solution
|