Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 2.1 (Objective)|10 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 2.2 (Objective)|9 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Fill In The Blanks)|1 VideosSOLID STATE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.2 (Objective)|9 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|2 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-SOLUTIONS-Exercises Archives (Subjective)
- What is the molarityk and molality of a 13% solution (by weight) of su...
Text Solution
|
- The vapour pressure of pure benzene is 639.7 mm Hg and the vapour pres...
Text Solution
|
- Two liquids A and B form an ideal solution. At 300 K, the vapour press...
Text Solution
|
- An organic compound C(x)H(2y)O(y) was burnt with twice the amount of o...
Text Solution
|
- The following statements is true only under some specific conditions. ...
Text Solution
|
- The vapour pressures of ethanol and methanol are 44.5 and 88.7 mm Hg, ...
Text Solution
|
- The vapour pressure of a dilute aqueous solution of glucosse (C(6)H(12...
Text Solution
|
- The vapour pressure of pure benzene at a certain temperature is 640 mm...
Text Solution
|
- The degree of dissociation of Ca(NO(3))(2) in a dilute aqueous solutio...
Text Solution
|
- The addition of 0.643 g of a compound to 50 mL of benzene (density 0.8...
Text Solution
|
- What weight of the non-volatile urea (NH2- CO - NH2) needs to be disso...
Text Solution
|
- A motor vehicle raditor was filled with 8 Lof water to which 2 Lof met...
Text Solution
|
- The molar volume of liquid benzene (density 0.877 g mL^(-1)) increases...
Text Solution
|
- A solution of a non-volatile solute in water freezes at -0.30^(@)C. Th...
Text Solution
|
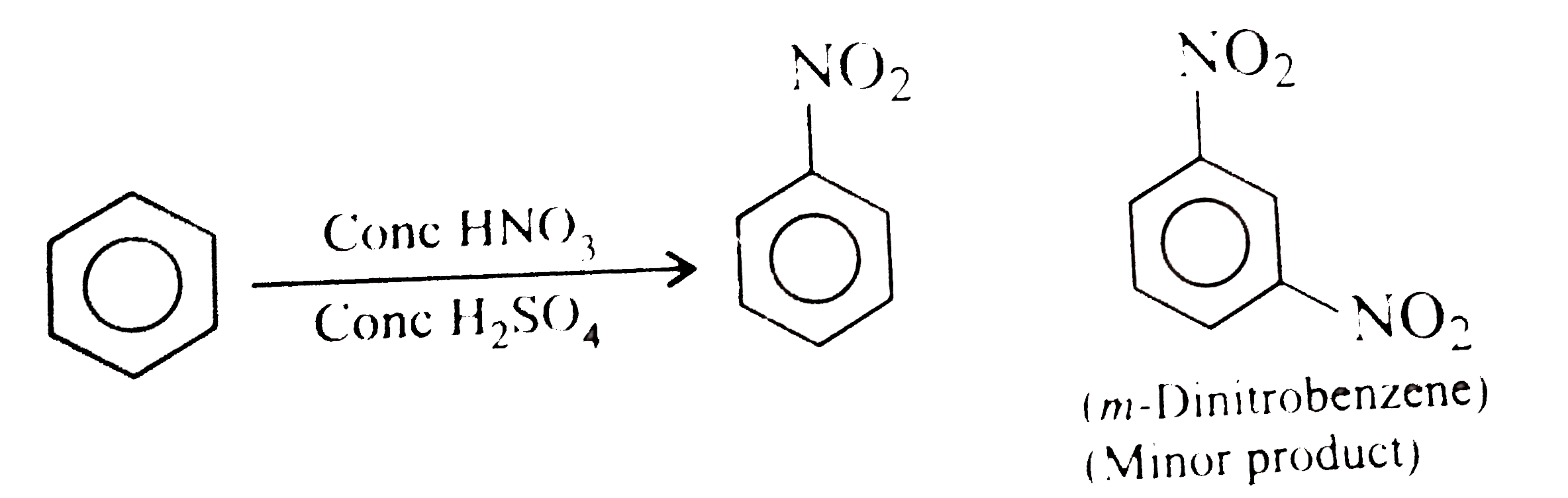
- Nirtobenzene is formed as the major product along with a minor product...
Text Solution
|
- To 500cm^(3) of water, 3.0 xx 10^(-3) kg acetic acid is added. If 23% ...
Text Solution
|
- When 1.22 g C(6)H(5)COOH is added into two solvents, the following dat...
Text Solution
|
- In an experiment, 72.5 g of C(6)H(5)OH (phenol) is dissolved in a solv...
Text Solution
|