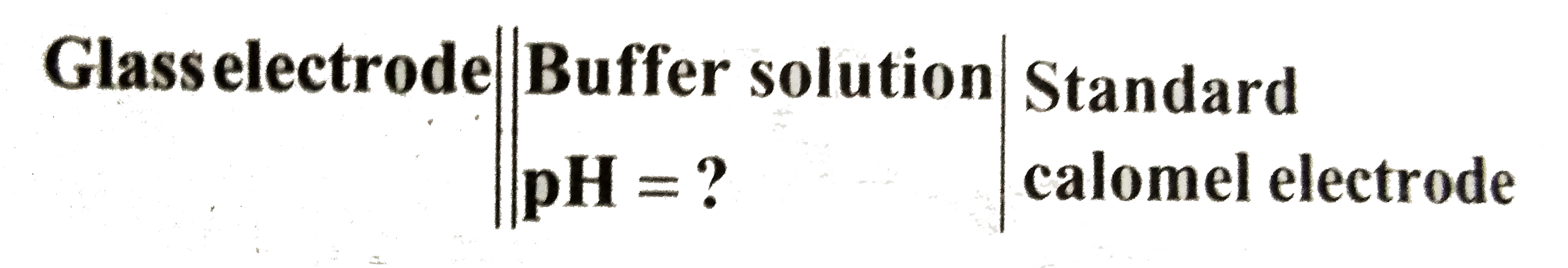Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples (Electrochemical Cell)|36 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Examples(Electrolysis And Electrolytic Cells)|12 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ELECTROCHEMISTRY-Archieves Subjective
- If the EMF of the above cell is 0.03V,E(SC E)=0.24V,E^(c-).(glass)=0.5...
Text Solution
|
- A current of 3.7 A is passed for 6hrs. Between Ni electrodes in 0.5 L ...
Text Solution
|
- Consider the cell : Zn|Zn^(2+)(aq)(1.0M)||Cu^(2+)(aq)(1.0M)||Cu Th...
Text Solution
|
- In an electrolysis experiment, current was passed for 5h through two c...
Text Solution
|
- How long a current of 3A has to be passed through a solution of silver...
Text Solution
|
- Give reasons in one or two sentences :" anhydrous HCl is a bad conduc...
Text Solution
|
- The EMF of the following cellis 1.05V at 25^(@)C: Pt,H(2)(g)(1.0 atm...
Text Solution
|
- During the discharge of a lead storage battery, the density of sulphur...
Text Solution
|
- A 100 watt, 110 volt incandescent lamp is connected in series with an ...
Text Solution
|
- A cell contains two hydrogen electrodes. The negative electrode is in ...
Text Solution
|
- In a fuel cell, hydrogen and oxygen react to produce electricity. In p...
Text Solution
|
- An acidic solution of Cu^(2+0 salt containing 0.4g of Cu^(2+) is elect...
Text Solution
|
- Define Photolytic decomposition reaction.
Text Solution
|
- The standard reduction potential of Cu^(2+)|Cu and Ag^(o+)|Ag electrod...
Text Solution
|
- Calculate the quantity of electricity that would be required to reduce...
Text Solution
|
- Zinc granules are added in excess to 500mL OF 1.0m nickel nitrate solu...
Text Solution
|
- During the electrolysis of an aqueoius nitric acid solution using pt e...
Text Solution
|
- The standard reduction potential of Ag^(+)//Ag electrode at 298 K is 0...
Text Solution
|
- An aqueous solution of NaCl on electrolysis gives H(2)(g), Cl(2)(g), a...
Text Solution
|
- The standard reduction potential for the half cell : NO(3)^(c-)(aq)+...
Text Solution
|
- Chromium metal is electroplated using an acidic solution containing Cr...
Text Solution
|