A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exerciseassertion -Reasoning|25 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exerciseinterger|8 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercisemultiple Correct Ansers|53 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ELECTROCHEMISTRY-Exercises Ingle Correct
- If E^(c-).(Fe^(3+)|Fe) and E^(c-).(Fe^(2+)|Fe) are =-0.36 V and -0.439...
Text Solution
|
- The standard electrode of a metal ion (Ag|Ag^(o+)) and metal - insolu...
Text Solution
|
- The standard reduction potential at 25 degree Celsius for the reactio...
Text Solution
|
- What would be the magnitude of EMF of the following cell: at 25^(...
Text Solution
|
- The rusting of iron takes place as follows : 2H^(o+)+2e^(-) +(1)/(2...
Text Solution
|
- For the electrolytic production of NaClO(4) from NaClO(3) according to...
Text Solution
|
- If the specific conductance of 1 M H(2)SO(4) solution is 26xx10^(-2)S ...
Text Solution
|
- The highest electrical conductivity of the following aqueous solutions...
Text Solution
|
- Which of the following statements is wrong ?
Text Solution
|
- Which of the following statement is correct ? (a)Specific conductance ...
Text Solution
|
- For a dilute solution of a strong electrolyte, the variation of molar ...
Text Solution
|
- How many coulombs are required for the following oxidation? 1 mole o...
Text Solution
|
- On electrolysis of a solution of dilute H(2)SO(4) between platinum ele...
Text Solution
|
- In electrolysis of very dilute of NaOH using platinum electrodes
Text Solution
|
- During the electrolysis of fused NaCl , which reaction occurs at anode...
Text Solution
|
- Two platinum electrodes were immersed in a solution of CuSO(4) and el...
Text Solution
|
- In an experiment setup for the measurement of EMF of a half cell using...
Text Solution
|
- The reference calomel electrode is made from which of the following ?
Text Solution
|
- When electricity is passed through a solution of AlCl(3) and 13.5g of ...
Text Solution
|
- What weight of copper will be deposited by passing 2 faradays of elect...
Text Solution
|
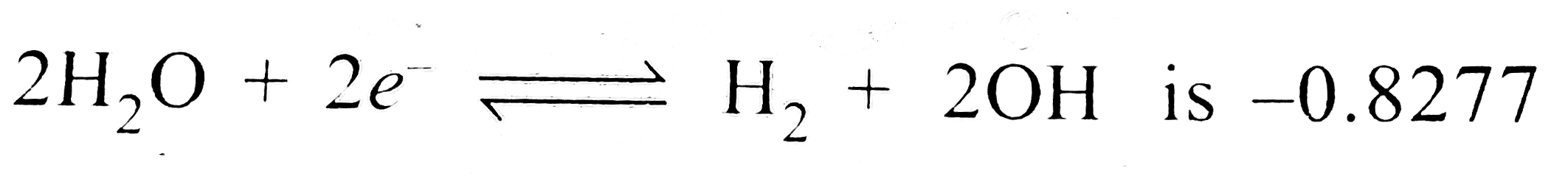 volt. The equilibrium constant for the reaction `:`
volt. The equilibrium constant for the reaction `:`  is
is