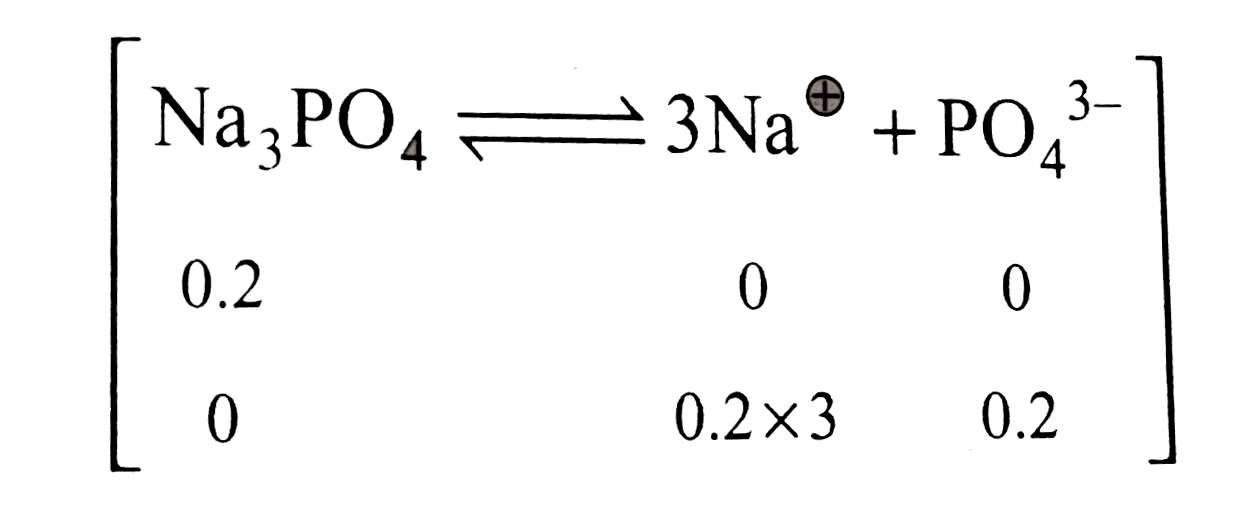
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exerciseassertion -Reasoning|25 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exerciseinterger|8 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercisemultiple Correct Ansers|53 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ELECTROCHEMISTRY-Exercises Ingle Correct
- 500mL of 1N solution of CuCl(2) was electrolyszed with a current fo 2 ...
Text Solution
|
- Ionic strength of 0.4 M CaCl(2) is
Text Solution
|
- Ionic strength of 0.4M CaCl(2) is
Text Solution
|
- Ionic strength of a solution made by mixing equal volumes of 0.01 M Na...
Text Solution
|
- Marshell's acid is prepared by the electrolytic oxidation of H(2)SO(4)...
Text Solution
|
- The volume of gases evolved at STP by passing 0.1A of current for 965s...
Text Solution
|
- Give an example of Strong acid and a Weak acid.
Text Solution
|
- The volume of gases evolved at STP by passing 0.2A of current for 965 ...
Text Solution
|
- The products obtained at cathode and anode on electrolysis of aqueous ...
Text Solution
|
- What is the electrode potential of a gasous hydrogen electrode dipped ...
Text Solution
|
- The EMF of concentration cell consisting of two zinc electrodes, one d...
Text Solution
|
- A certain electrode has standard ( reduction potential ) of 0.384 V. T...
Text Solution
|
- The potential of a hydrogen electrode in a solution with pOH=4 at 25^(...
Text Solution
|
- How much will the reduction potential of a hydrogen electrode change w...
Text Solution
|
- Consider the electrode Ag|AgCl(s),Cl^(-)(0.1M),i.e., silver electrode ...
Text Solution
|
- A current strength of 1.0A is passed for 96.5 s through 100mL of a sol...
Text Solution
|
- A solution turns blue litmus red. What would be its rough pH value? (...
Text Solution
|
- The standard EMF of decinormal calomel electrode is 0.268 V. The EMF i...
Text Solution
|
- The standard EMF of quinhydrone is 0.699V. The EMF of the quinhydrone ...
Text Solution
|
- A hydrogen electrode placed in a solution containing sodium acetate an...
Text Solution
|