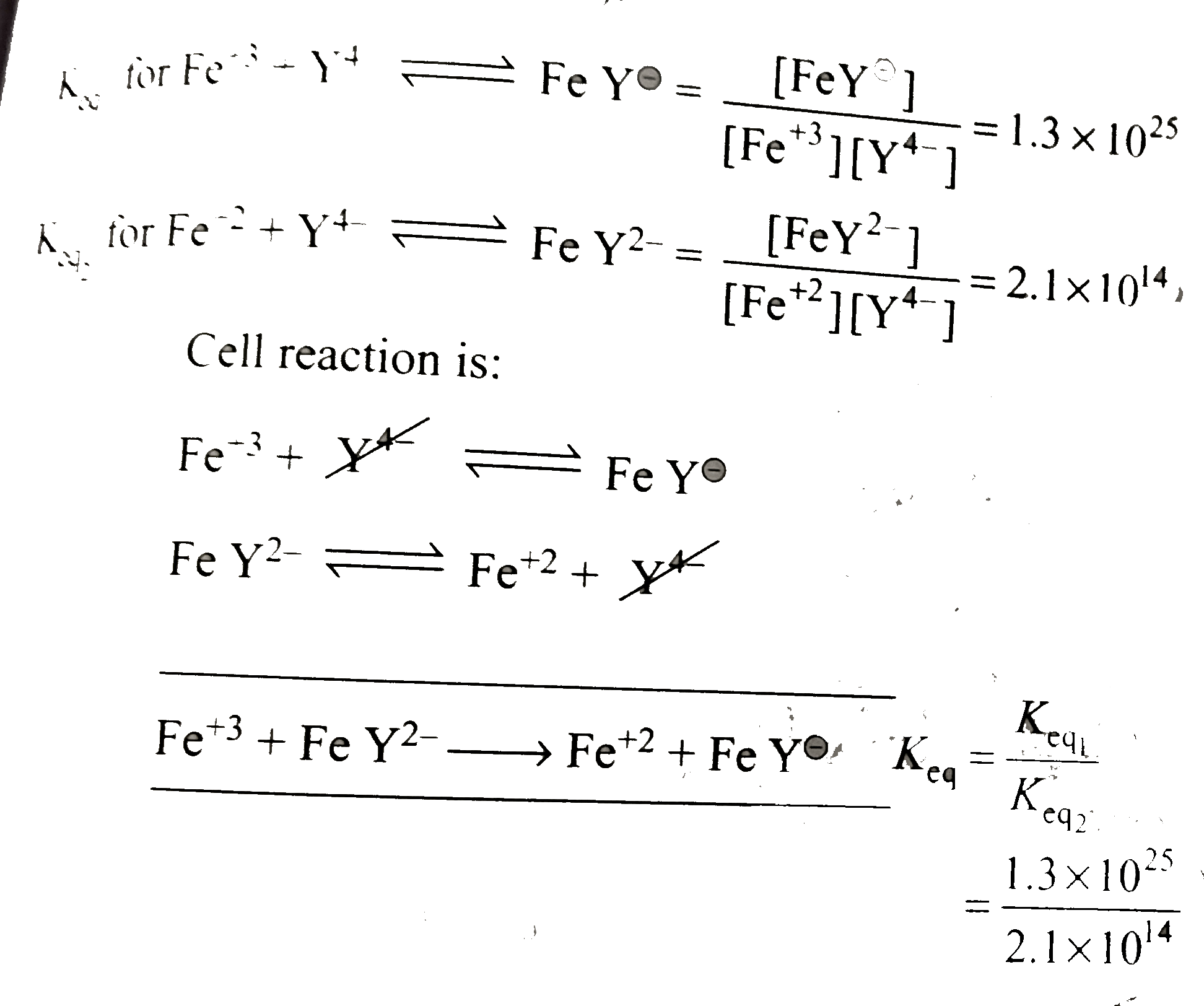A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exerciseassertion -Reasoning|25 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exerciseinterger|8 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercisemultiple Correct Ansers|53 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ELECTROCHEMISTRY-Exercises Ingle Correct
- The electricity conductivity of a solution serves as a means of determ...
Text Solution
|
- A constant current was passed through a solution of AuCl(4)^(c-) ion b...
Text Solution
|
- E^(c-) for FeY^(c-)+e^(c-) rarr FeY^(2-) given: Fe+3/+2=0.77V (a)0.13...
Text Solution
|
- Calculate E^(c-) for the reactions : ZnY^(2-)hArrZn(s)+Y^(4-) where...
Text Solution
|
- The solubility product of Pb(3)(AsO(4))(2) is 4.1xx10^(-36). E^(c-) fo...
Text Solution
|
- A cell is to be constructed to show a redox change : Cr+2Cr^(3+)hArr...
Text Solution
|
- E^(c-) for Cr^(3+)+3e^(-) rarr Cr and Cr^(3+)+e^(-) rarr Cr^(2+) are -...
Text Solution
|
- The efficiency of a fuel cell si 80% and the standard heat of reaction...
Text Solution
|
- The E(cell) for a given cell is 1.2346 and 1.2340V at 300K and 310K, ...
Text Solution
|
- A curent of 3A was passed for 1 hour through an electrolyte solution o...
Text Solution
|
- The E^(c-) for Cu^(2+)//Cu^(o+),Cu^(o+)//Cu,Cu^(2+)//Cu, are 0.15V,0.5...
Text Solution
|
- Total charge required to convert three moles of Mn(2)O(4) to MnO(4)^(c...
Text Solution
|
- A current of 965A is passed for 1 s through 1L solution of 0.02N NiSO(...
Text Solution
|
- For the given cell Pt(D(2)|D^(o+))||H^(o+)|Pt(H(2)), if E^(c-).(D(2)|D...
Text Solution
|
- What is E^(c).(red) for the reaction: Cu^(2+)+2e^(-) rarr Cu in the ha...
Text Solution
|
- The combustion of butane in O(2) at 1 bar and 298K shows a decrease in...
Text Solution
|
- A half cell reaction Ag(2)S(s)+2e^(-) rarr 3Ag(s)+S^(2-) is carried ou...
Text Solution
|
- Which one is wrong if electrolysis of CH(3)COONa(aq) is made using Pt ...
Text Solution
|
- The calomel and quinhydrone electrodes are reversible with respect to ...
Text Solution
|
- The EMF of Ni-Cad battery is dependent of :
Text Solution
|