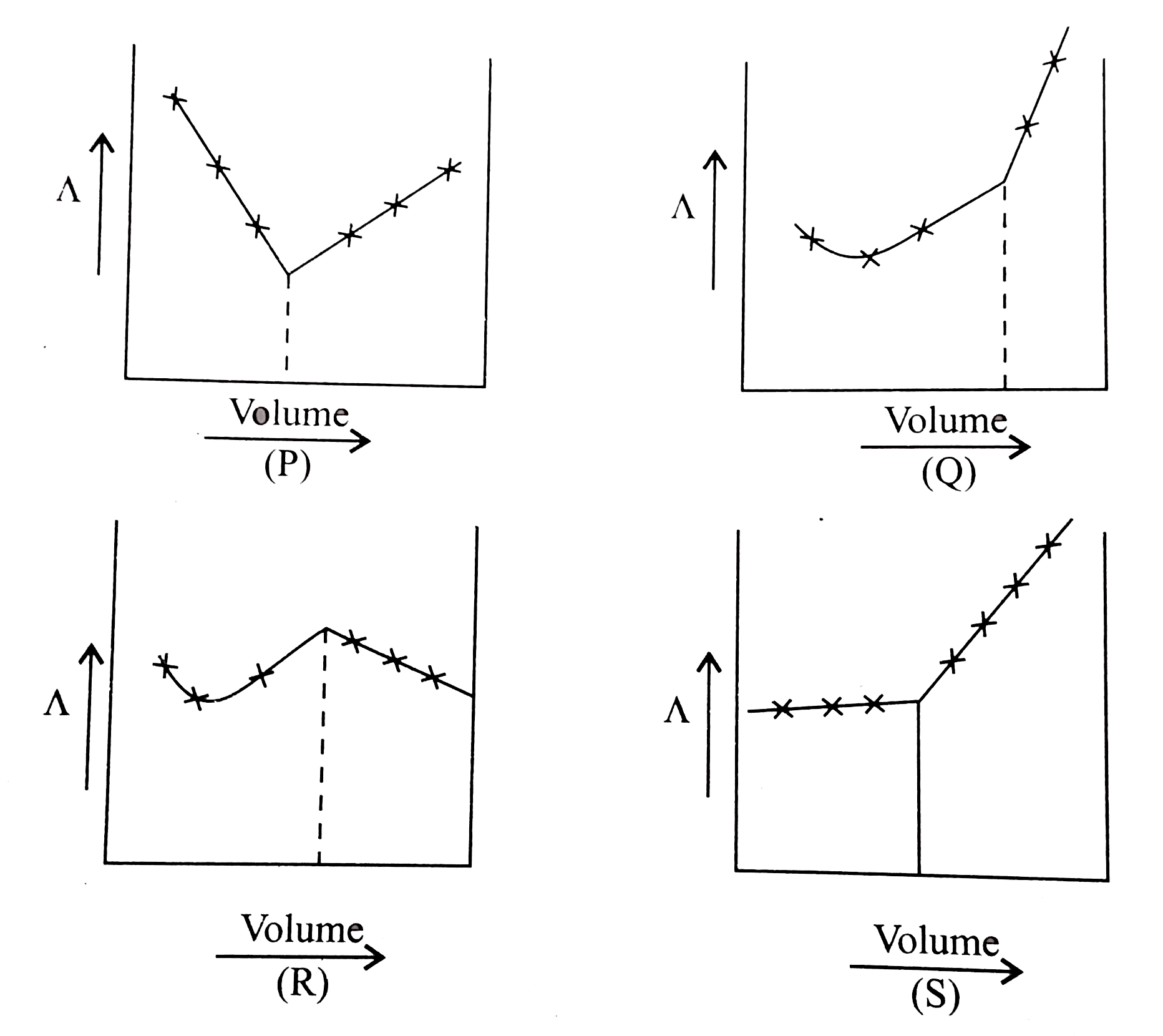
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archieves Fill In The Blanks|4 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archieves True/False|1 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archieves Multiple Correct Ansers|2 VideosD AND F BLOCK ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|29 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|14 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ELECTROCHEMISTRY-Archieves Single Correct
- When a lead storage battery is discharged,
Text Solution
|
- The standard reductino potentials E^(c-) for the half reactinos are as...
Text Solution
|
- The standard reduction potentials of Cu^(2+)|Cu and Cu^(2+)|Cu^(o+) ar...
Text Solution
|
- Standard electrode potential of three metals X, Y and Z are -1.2V,+0.5...
Text Solution
|
- The gasX at 1 atm is bubbled through a solution containing a mixture o...
Text Solution
|
- For the electrochemical cell, (M|M^(o+))||(X^(c-)|X),E^(c-).((M^(o+)|M...
Text Solution
|
- Arrange the following compounds in the order of increasing conductanc...
Text Solution
|
- A standard solution of KNO(3) is used to make salt bridge, because
Text Solution
|
- Standard electrode potential data are useful for understanding the sui...
Text Solution
|
- Define Reduction in terms of Hydrogen and Oxygen.
Text Solution
|
- Zn|Zn^(2+)(a=0.1M)||Fe^(2+)(a=0.01M)Fe. The EMF of the above cell i...
Text Solution
|
- The rusting of iron takes place as follows : 2H^(o+)+2e^(-) +(1)/(2...
Text Solution
|
- Electrolysis of dilute aqueous NaCl solution was carried out by passin...
Text Solution
|
- Consider the following cell reation : 2Fe(s)+O(2)(g)+4H^(o+)(aq) rar...
Text Solution
|
- AgNO(3)(aq) was added to an aqueous KCl solution gradually and the con...
Text Solution
|
- given E(S(2)O(8)^(2-)//SO(4)^(2-)^(@)=2.05V E(Br(2)//Br^(-))^(@)=1.4...
Text Solution
|
- The metal that cannot obtained by electrolysis of an aqueous solution ...
Text Solution
|
- Resistance of a conductivity cell filled with 0.1 mol L^(-1) KCl solut...
Text Solution
|
- The equivalent conductance of NaCl at concentration C and at infinite...
Text Solution
|
- Find the standard electrode potential of MnO(4)^(c-)|MnO(2). The stand...
Text Solution
|