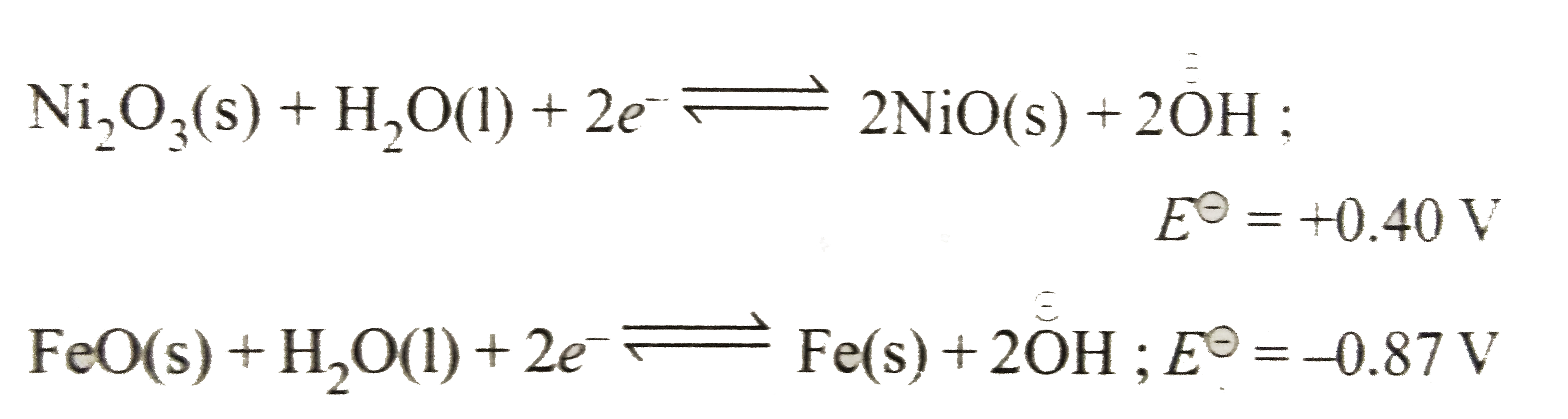Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-ELECTROCHEMISTRY-Archieves Subjective
- During the electrolysis of an aqueoius nitric acid solution using pt e...
Text Solution
|
- The standard reduction potential of Ag^(+)//Ag electrode at 298 K is 0...
Text Solution
|
- An aqueous solution of NaCl on electrolysis gives H(2)(g), Cl(2)(g), a...
Text Solution
|
- The standard reduction potential for the half cell : NO(3)^(c-)(aq)+...
Text Solution
|
- Chromium metal is electroplated using an acidic solution containing Cr...
Text Solution
|
- The standard reduction potential of the Ag^(o+)|Ag electrode at 298K i...
Text Solution
|
- The Edison storage cell is represented as : Fe(s)|FeO(s)|KOH(aq)|Ni(...
Text Solution
|
- An excess of liquid mercury is added to an acidicfied solution of 1.0x...
Text Solution
|
- The standard reduction potential for Cu^(2+)|Cu is +0.34V. Calculate t...
Text Solution
|
- How many grams of silver could be plated out on a serving tray by the ...
Text Solution
|
- Calculate the equilibrium constant for the reaction : Fe^(2+)+Ce^(4+)h...
Text Solution
|
- Calculate the equilibrium constant for the reaction, 2Fe^(3+) + 3I^(-)...
Text Solution
|
- Find the solubility product of a saturated solution of Ag(2)CrO(4) in ...
Text Solution
|
- A cell, Ag|Ag^(o+)||Cu^(2+)|Cu , initially contains 1 M Ag^(o+) and 1M...
Text Solution
|
- Copper sulphate solution (250 ML) was electrolyzed using a platinum an...
Text Solution
|
- The following electrochemical cell has been set up Pt(1)|Fe^(3+)|Fe^...
Text Solution
|
- The standard potential of the following cell is 0.23V at 15^(@)C and 0...
Text Solution
|
- Two students use same stock solution of ZnSO(4) and a solution of CuSO...
Text Solution
|
- The equilibrium constant of the reaction; Cu(s)+2Ag + (aq)⟶Cu 2+ (...
Text Solution
|
- We have taken a saturated solution of AgBr, whose K(sp) is 12xx10^(-14...
Text Solution
|