Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 4.1 (Objective)|9 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 4.2 (Objective)|10 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|23 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL KINETICS-Solved Example
- The rate of reaction: H(2)O + 2S(2)O(4)^(2-)(aq) rarr 2HSO(3)^(ɵ)(aq...
Text Solution
|
- The experiment data for the decompoistion of nitrogen pentoxide in the...
Text Solution
|
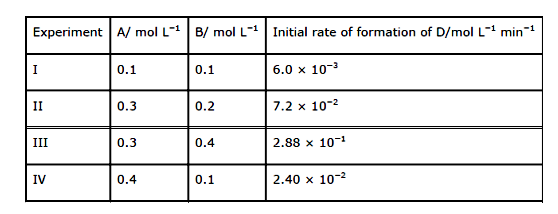
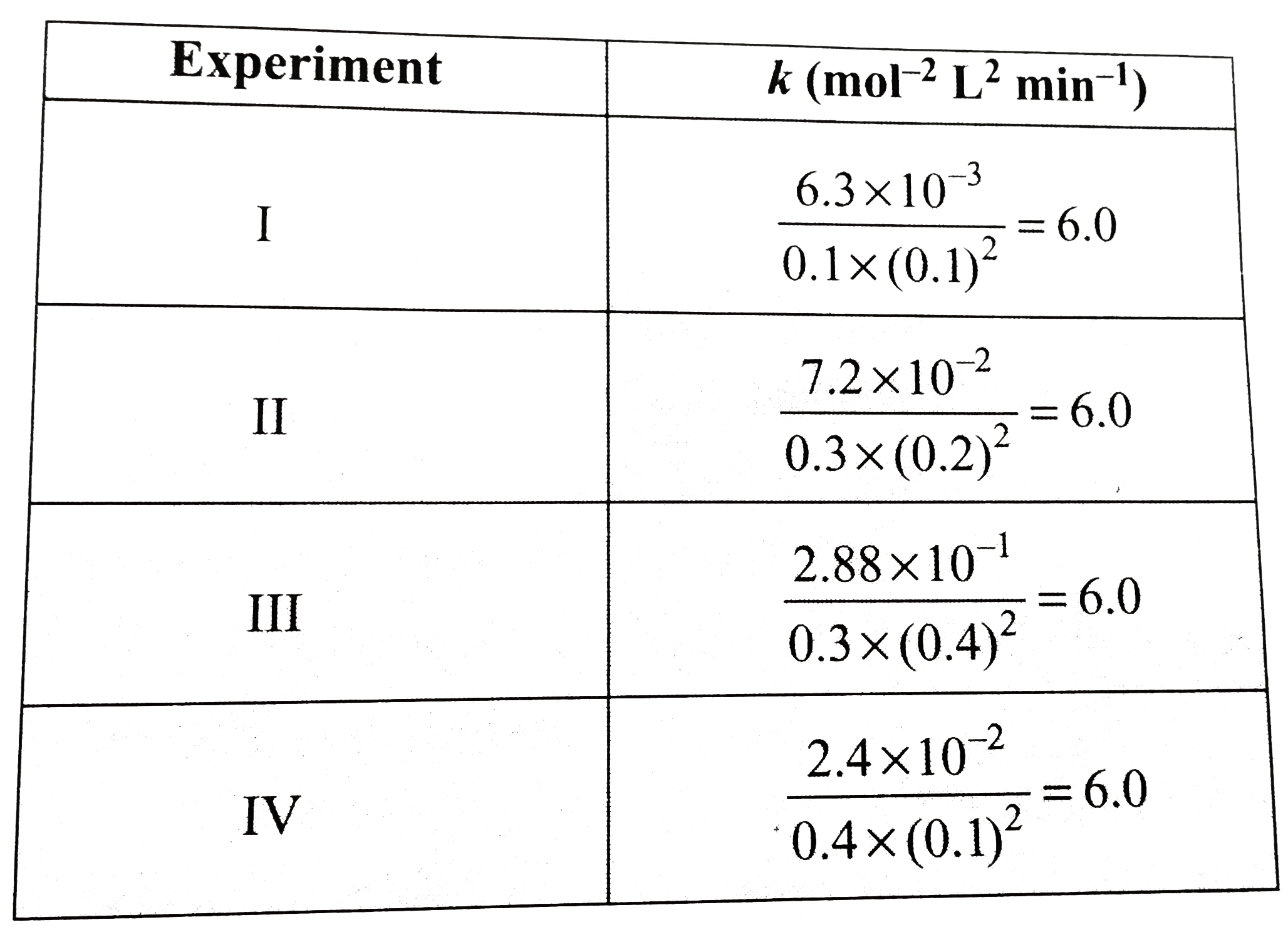
- The following results have been obtained during the kinetic studies of...
Text Solution
|
- What is the ratio of t(1//2) to t(1//3) (for the amount of substance l...
Text Solution
|
- The half life for a given reaction was doubled as the initial concentr...
Text Solution
|
- Why the term half life is not used for second order reaction?
Text Solution
|
- The reaction SO(2)Cl(2) overset(k(1))rarr SO(2)+Cl(2) is a first order...
Text Solution
|
- A first order reaction has specific reaction rate of 10^(-3) s^(-1) . ...
Text Solution
|
- The esterification of acetic anhydride by ethyl alcohol can be represe...
Text Solution
|
- An optically active drug has one chiral centre and only dextro rotator...
Text Solution
|
- A radioactive element (t(1//2) = 50 days) is spread over a room. Its a...
Text Solution
|
- .(84)Po^(218) (t(1//2) = 3.05 min) decays to .(82)Pb^(214) (t(1//2) = ...
Text Solution
|
- The complex [Co(NH(3))(5)F]^(2+) reacts with water according to the eq...
Text Solution
|
- For the reaction k(1) = 1.78 xx 10^(-3)s^(-1) and k(2) = 5.8 xx 1...
Text Solution
|
- Substance A isomerizes according to a first order rate law with k = 5....
Text Solution
|
- A certain reaction A + B rarr Products is first order w.r.t. each reac...
Text Solution
|
- Balance the reaction when Nitrogen reacts with Hydrogen to form ammoni...
Text Solution
|
- Sodium reacts with dilute hydrochloric acid. Name the gas evolved.
Text Solution
|
- In a certain polluted atmosphere containing O(3) at a steady-state con...
Text Solution
|
- Write a rate expresison for each speices. Assuming [B] is constant, wr...
Text Solution
|