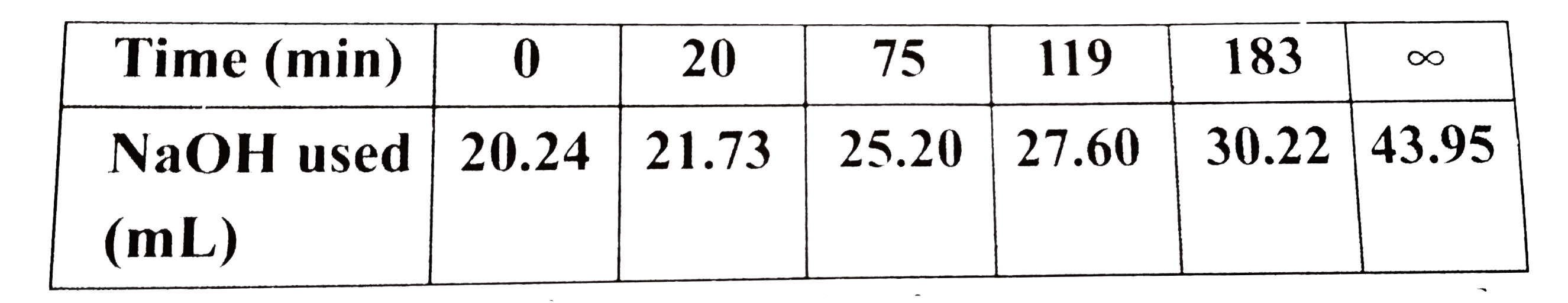
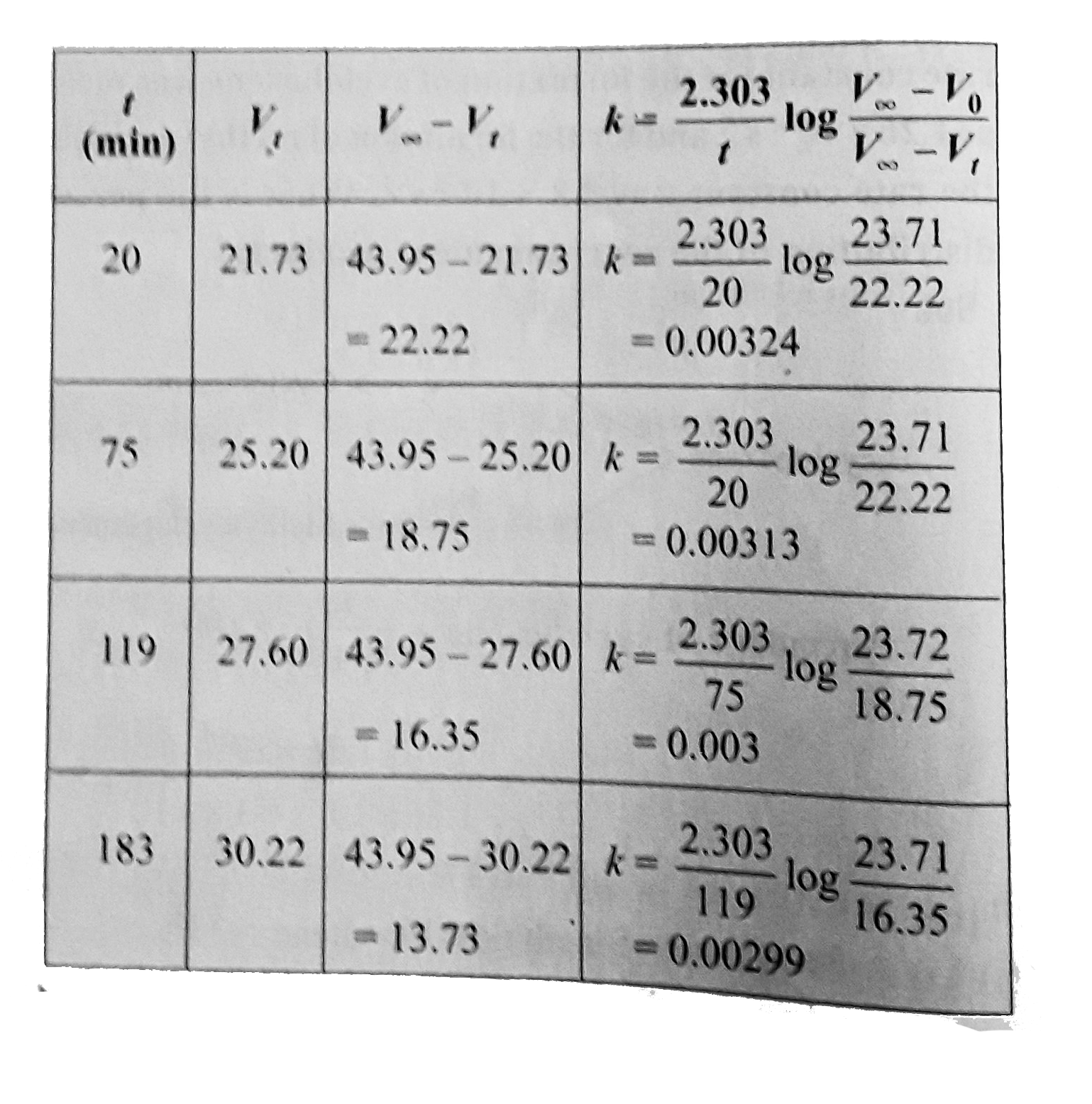
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 4.1 (Objective)|9 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 4.2 (Objective)|10 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|23 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL KINETICS-Solved Example
- A proposed mechanism for a reaction ArarrB is A underset(k(2))overse...
Text Solution
|
- Bicyclohexane was found to undergo two parallel first order rearrangem...
Text Solution
|
- Prepare ethanol from ethylamine and formaldehyde.
Text Solution
|
- The decompoistion of Cl(2)O(7) at 400 K in gas phase to Cl(2) and O(2)...
Text Solution
|
- Ethylene is profuced by C(4)H(8)("cyclobutane")overset(Delta)rarr2C(...
Text Solution
|
- form the following data for the decompoistion of N(2)O(5) in carbon te...
Text Solution
|
- 1.0 mL of ethyl acetate was added to 25 mL of N//2 HCl, 2 mL of the mi...
Text Solution
|
- The inverison of cane sugar was studied is HCl at 298 K. The following...
Text Solution
|
- An exothermic reaction XrarrY has an activation energy of 90kJ mol^(-1...
Text Solution
|
- Refer picture given below. (a) Calculate DeltaH for the reaction and...
Text Solution
|
- Calculate the activation energy of a reaction whose reaction rate at 3...
Text Solution
|
- The activation energy of a reaction is 94.14 kJ mol^(-1) and the value...
Text Solution
|
- The rate constant of a reaction is 1.5xx10^7 s^(-1) " at" 50^@C and 4...
Text Solution
|
- Convert propane into prop-2-en-1-ol
Text Solution
|
- For two firstorder reactions having same concentration of A and B at t...
Text Solution
|
- The Arrehenius equation for the rate constant of decompoistion of meth...
Text Solution
|
- The activation energy of the reaction: A + B rarr Products is 105.73 k...
Text Solution
|
- For a reverisble reaction A hArr B, if pre-exponential factor is same...
Text Solution
|
- Two reaction : X rarr Products and Y rarr Products have rate constants...
Text Solution
|
- The decompoistion of compound A in solution is a first order process w...
Text Solution
|