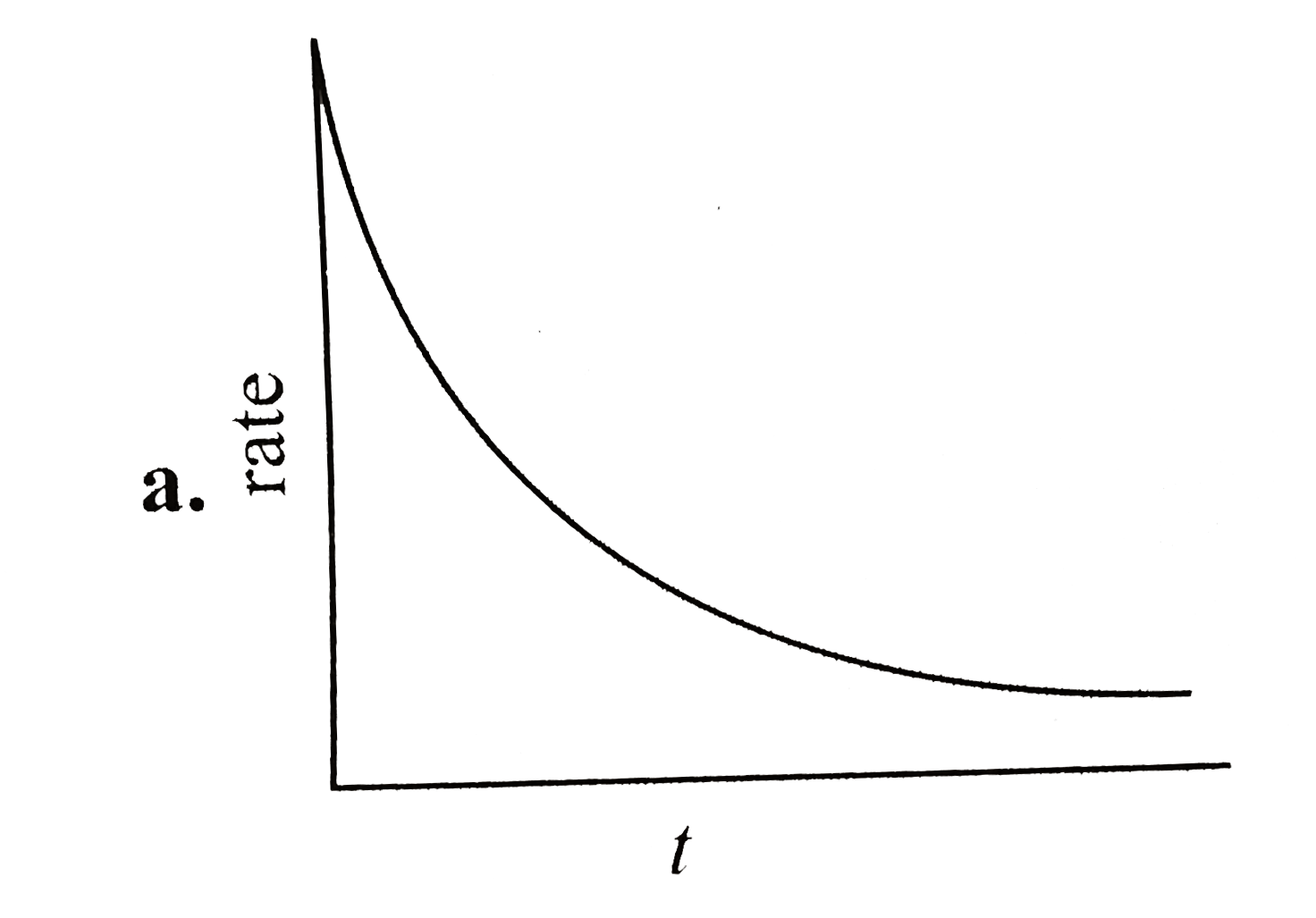
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex4.4 Objective|10 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Linked Comprehension|59 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 4.3 (Objective)|15 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL KINETICS-Ex 4.3 More Than One Correct
- Which of the following is/are correct about the first order reaction ?
Text Solution
|
- A reaction is 10% complete in 5min and 50% complete in 25 min. Which o...
Text Solution
|
- The correct nature of plot for first order reaction is (are):
Text Solution
|
- The correct statement (s) are
Text Solution
|
- For zero order reaction which is (are) true?
Text Solution
|