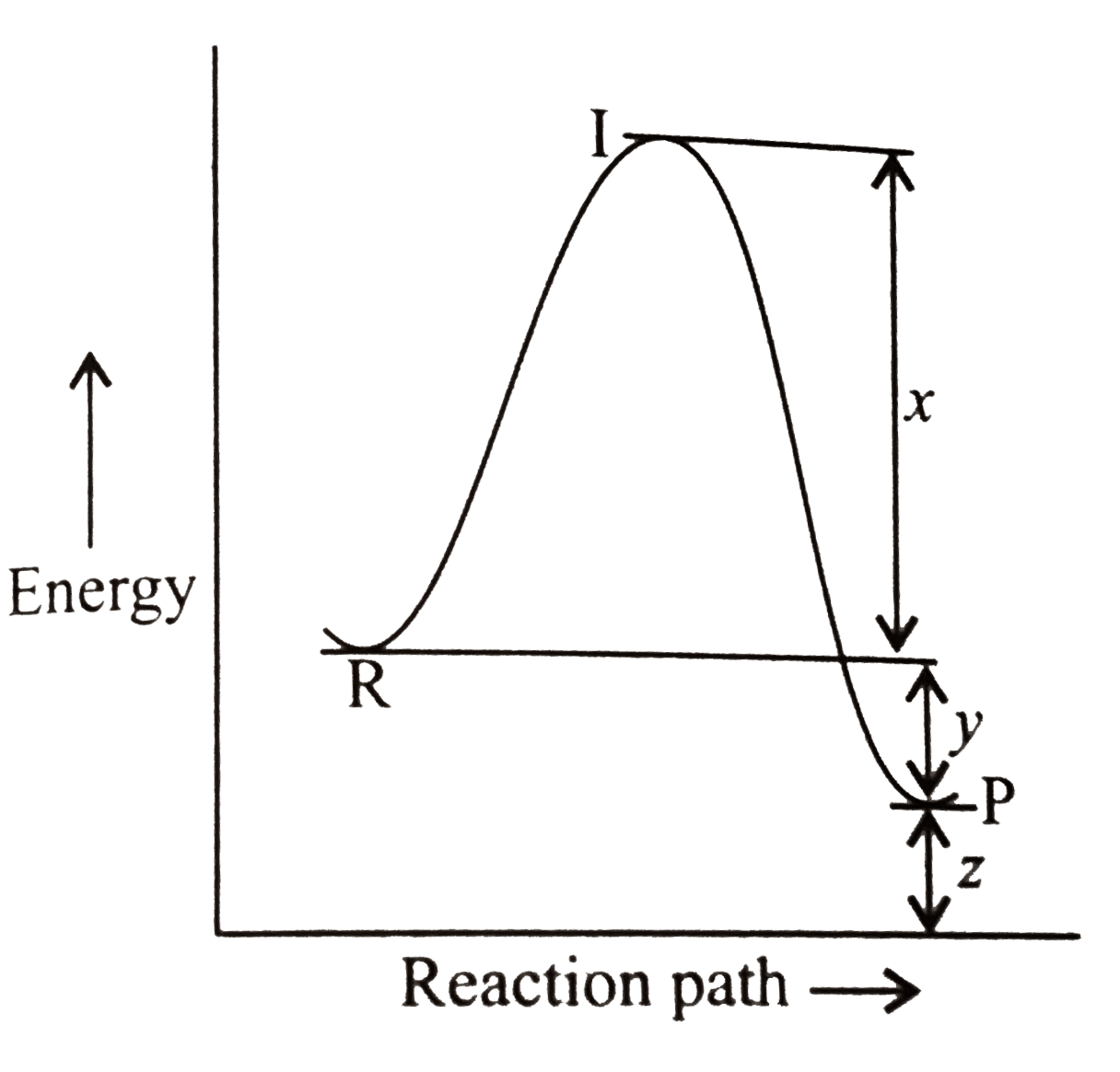
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|33 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|177 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex4.4 Objective|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL KINETICS-Exercises Linked Comprehension
- The energy profile diagram for the reaction: CO(g)+NO(2)(g) hArr CO(...
Text Solution
|
- The energy profile diagram for the reaction: CO(g)+NO(2)(g) hArr CO(...
Text Solution
|
- The energy profile diagram for the reaction: CO(g)+NO(2)(g) hArr CO(...
Text Solution
|
- The reaction S(2)O(8)^(2-) + 3I^(ɵ) rarr 2SO(4)^(2-) + I(3)^(ɵ) is of ...
Text Solution
|
- The reaction S(2)O(8)^(2-) + 3I^(ɵ) rarr 2SO(4)^(2-) + I(3)^(ɵ) is of ...
Text Solution
|
- Conisder the reaction represented by the equation: CH(3)Cl(g) + H(2)...
Text Solution
|
- Conisder the reaction represented by the equation: CH(3)Cl(g) + H(2)...
Text Solution
|
- Conisder the reaction represented by the equation: CH(3)Cl(g) + H(2)...
Text Solution
|
- Conisder the reaction represented by the equation: CH(3)Cl(g) + H(2)...
Text Solution
|
- Conisder the reaction represented by the equation: CH(3)Cl(g) + H(2)...
Text Solution
|
- For the reaction: aA + bB rarr cC+dD Rate = (dx)/(dt) = (-1)/(a)(d[A...
Text Solution
|
- For the reaction: aA + bB rarr cC+dD Rate = (dx)/(dt) = (-1)/(a)(d[A...
Text Solution
|
- For the reaction: aA + bB rarr cC+dD Rate = (dx)/(dt) = (-1)/(a)(d[A...
Text Solution
|
- For the reaction: aA + bB rarr cC+dD Rate = (dx)/(dt) = (-1)/(a)(d[A...
Text Solution
|
- The energy change accompanying the equilibrium reaction A hArr B is -3...
Text Solution
|
- The energy change accompanying the equilibrium reaction A hArr B is -3...
Text Solution
|
- The energy change accompanying the equilibrium reaction A hArr B is -3...
Text Solution
|
- In the start of summer, a given sample of milk turns sour at room temp...
Text Solution
|
- In the start of summer, a given sample of milk turns sour at room temp...
Text Solution
|
- In the start of summer, a given sample of milk turns sour at room temp...
Text Solution
|