A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|33 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|177 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex4.4 Objective|10 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL KINETICS-Exercises Linked Comprehension
- For the reaction: aA + bB rarr cC+dD Rate = (dx)/(dt) = (-1)/(a)(d[A...
Text Solution
|
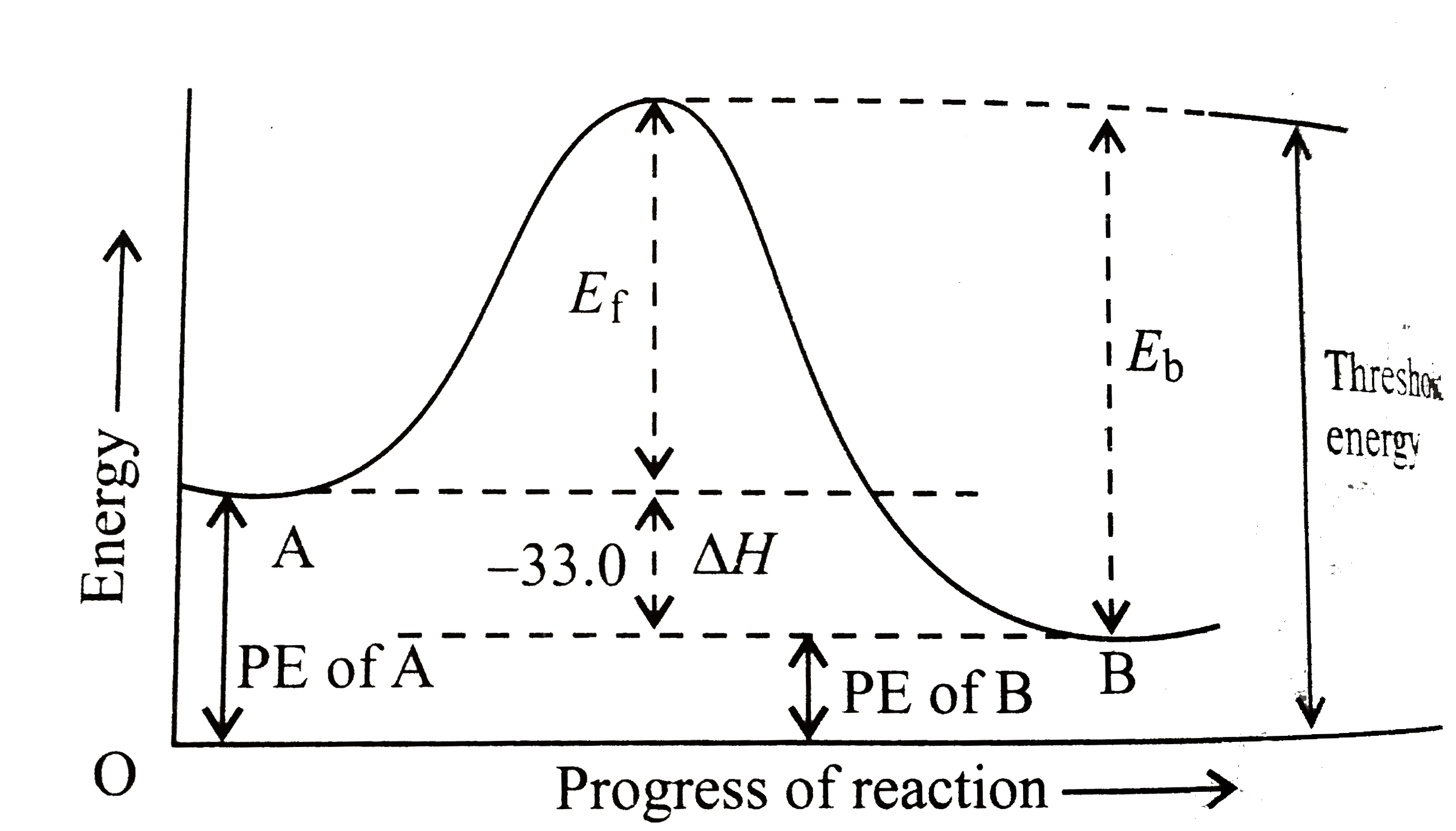
- The energy change accompanying the equilibrium reaction A hArr B is -3...
Text Solution
|
- The energy change accompanying the equilibrium reaction A hArr B is -3...
Text Solution
|
- The energy change accompanying the equilibrium reaction A hArr B is -3...
Text Solution
|
- In the start of summer, a given sample of milk turns sour at room temp...
Text Solution
|
- In the start of summer, a given sample of milk turns sour at room temp...
Text Solution
|
- In the start of summer, a given sample of milk turns sour at room temp...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- A colliison between reactant molecules must occur with a certain minim...
Text Solution
|
- The order of reaction is an experimentally determined quanity. It may ...
Text Solution
|
- The order of reaction is an experimentally determined quanity. It may ...
Text Solution
|
- The order of reaction is an experimentally determined quanity. It may ...
Text Solution
|
- The order of reaction is an experimentally determined quanity. It may ...
Text Solution
|
- The order of reaction is an experimentally determined quanity. It may ...
Text Solution
|
- Conisder the following elementary reaction, 2A + B + C rarr Products...
Text Solution
|
- Conisder the following elementary reaction, 2A + B + C rarr Products...
Text Solution
|