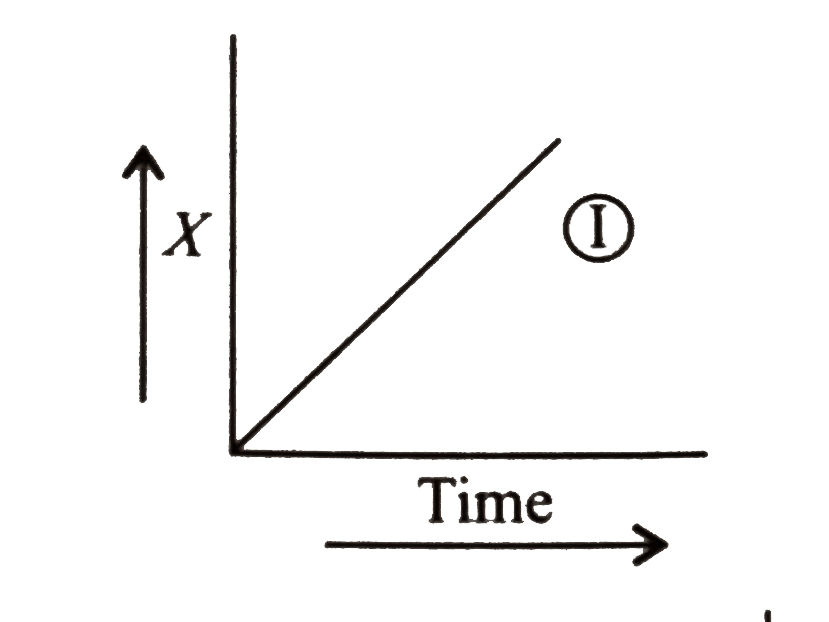
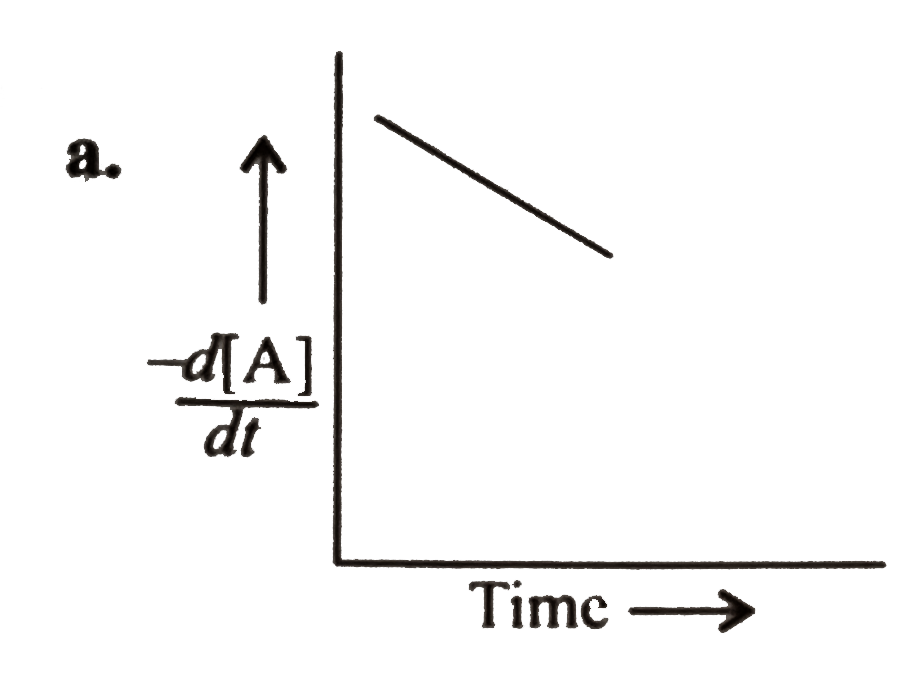
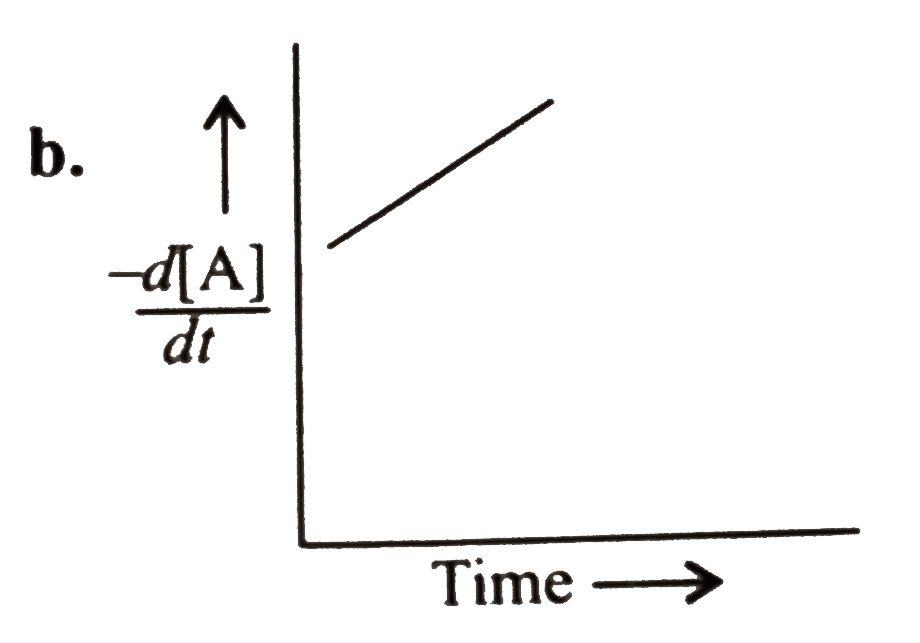
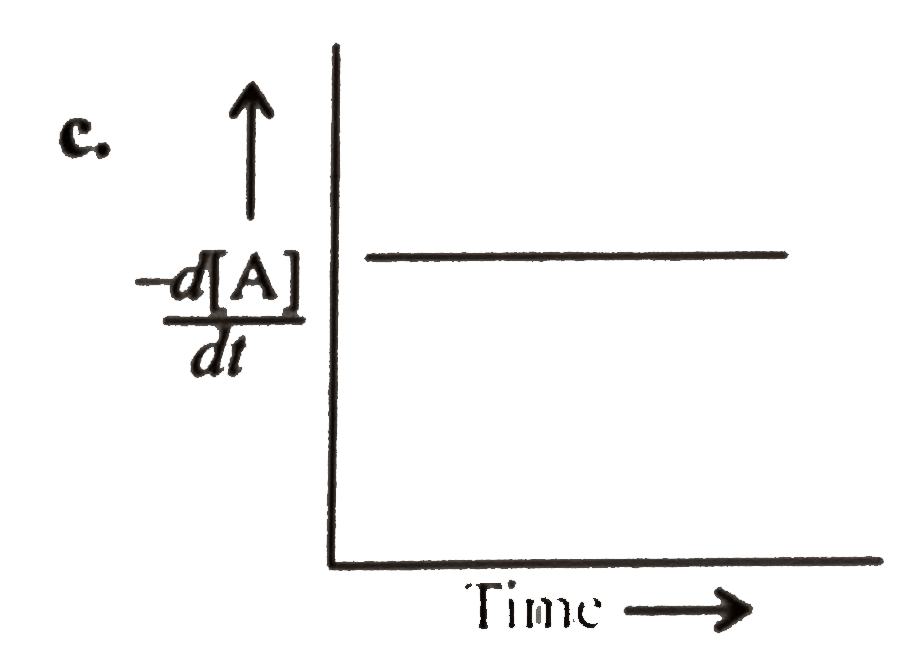
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Reasoning|22 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Integer|15 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|33 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL KINETICS-Exercises Single Correct
- Rate constant k = 1.2 xx 10^(3) mol^(-1) L s^(-1) and E(a) = 2.0 xx 10...
Text Solution
|
- The rate constant of a reaction is 0.0693 min^(-1). Starting with 10 m...
Text Solution
|
- The graph between concentration (X) of the Product and time of the rea...
Text Solution
|
- Following is the graph between log T(50) and log a (a = initial concen...
Text Solution
|
- The half life of radioactive element is 20 min. The time interval betw...
Text Solution
|
- Which of the following reaction is not of the first order ?
Text Solution
|
- For the reaction X+3Y rarrZ, which form of differential rate law is in...
Text Solution
|
- 60% of a first order reaction was completed in 60 min. The time taken ...
Text Solution
|
- 70% of a first order reaction was completed in 70 min. What is the hal...
Text Solution
|
- 80% of a fisrt order reaction was completed in 70 min. How much it wil...
Text Solution
|
- 90% of a first order reaction was completed in 100 min. How much time ...
Text Solution
|
- 90% of a first order reaction was completed in 100 min.What is the hal...
Text Solution
|
- The hydrolyiss of ester in alkaline medium is a
Text Solution
|
- A second order reaction requires 70 min to change the concentration of...
Text Solution
|
- The data for the reaction: A + B overset(k)rarr C. |{:("Experiment",...
Text Solution
|
- The temperature at which the average speed of perfect gas molecules is...
Text Solution
|
- The temperature at which the average speed of perfect gas molecules is...
Text Solution
|
- A catalyst lowers the activation energy of a reaction form 20 kJ mol^(...
Text Solution
|
- The rate of a reaction increases four-fold when the concentration of r...
Text Solution
|
- Two substances A and B are present such that [A(0)]=4[B(0] and half-l...
Text Solution
|