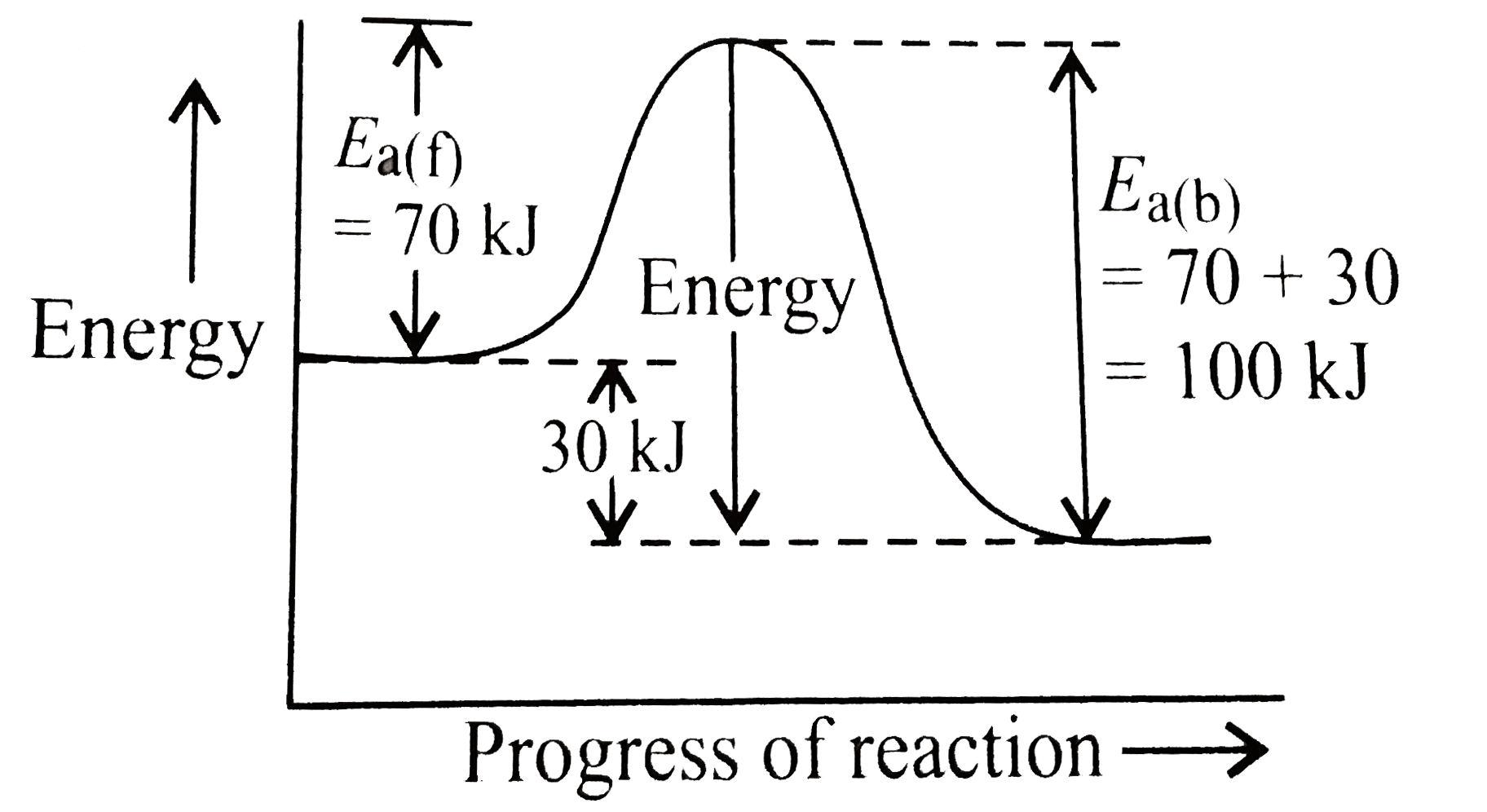
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Reasoning|22 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Integer|15 VideosCHEMICAL KINETICS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|33 VideosCARBOXYLIC ACIDS AND THEIR DERIVATIVES
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Analytical And Descriptive)|34 VideosCOORDINATION COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|18 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL KINETICS-Exercises Single Correct
- In a reaction carried out at 500 K, 0.001% of the total number of coll...
Text Solution
|
- A substance decomposes by following first order kinetics. If 50% of th...
Text Solution
|
- If a reaction A+BrarrC is exothermic to the extent of 30 KJ mol^(-1), ...
Text Solution
|
- The rate constant, activation energy, and Arrphenius parameter of a ch...
Text Solution
|
- The reaction A(g) + 2B(g) rarr C(g) + D(g) is an elementary process. I...
Text Solution
|
- A catalyst decreases E(a) form 100 KJ mol^(-1) to 80 KJ mol^(-1). At w...
Text Solution
|
- Which of the following graphs is for a second order reaction?
Text Solution
|
- The accompanying figure depicts a change in concentration of species A...
Text Solution
|
- Which of the following is correct graph for the reaction?
Text Solution
|
- Which of the following graphs represents zero order if A rarr P At ...
Text Solution
|
- Which of the following expresisons give the effect of temperature on t...
Text Solution
|
- The plot og log k vs 1//T helps to calculate
Text Solution
|
- For a first order reaction t(0.75) is 1386 s. Therefore, the specific ...
Text Solution
|
- In a first order reaction, the concentration of the reactant decreases...
Text Solution
|
- The rate equation for the second order reaction 2A+B rarr C is found t...
Text Solution
|
- A graph plotted between log k versus 1//T for calculating activation e...
Text Solution
|
- The potential energy diagram for a reaction R rarr P is given below. D...
Text Solution
|
- the activation energy for a simple chemical reaction A to B is...
Text Solution
|
- the reaction A to B follows first order Kinetics the time taken...
Text Solution
|
- Write the expression for quantum yield in photosynthesis.
Text Solution
|