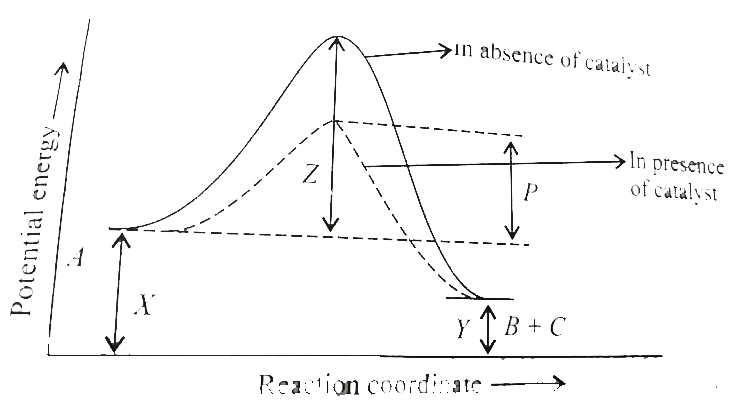
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|25 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|52 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Subjective|10 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 2.3 (Objective)|9 VideosSYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion Reasoning|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-SURFACE CHEMISTRY-Exercises Link Comprehension
- Adsorption is the tendency of accumulation of molecular species at the...
Text Solution
|
- Substances which alter the velocity of a reaction by mere presence, wi...
Text Solution
|
- Substances which alter the velocity of a reaction by mere presence, wi...
Text Solution
|
- Substances which alter the velocity of a reaction by mere presence, wi...
Text Solution
|
- Substances which alter the velocity of a reaction by mere presence, wi...
Text Solution
|
- Only the surface atoms in an adsorbent play an active role in adsorpti...
Text Solution
|
- A chemist studied the phenomenon of adsorption by putting blood charco...
Text Solution
|
- A chemist studied the phenomenon of adsorption by putting blood charco...
Text Solution
|
- Only the surface atoms in an adsorbent play an active role in adsorpti...
Text Solution
|
- Only the surface atoms in an adsorbent play an active role in adsorpti...
Text Solution
|
- Emulsions are also called the colloidal solutions in which the dispers...
Text Solution
|
- Emulsions are also called the colloidal solutions in which the dispers...
Text Solution
|
- Emulsions are also called the colloidal solutions in which the dispers...
Text Solution
|
- Emulsions are also called the colloidal solutions in which the dispers...
Text Solution
|
- Emulsions are also called the colloidal solutions in which the dispers...
Text Solution
|
- There are certain substances which behave as normal, strong electrolyt...
Text Solution
|
- There are certain substances which behave as normal, strong electrolyt...
Text Solution
|
- There are certain substances which behave as normal, strong electrolyt...
Text Solution
|
- There are certain substances which behave as normal, strong electrolyt...
Text Solution
|
- There are certain substances which behave as normal, strong electrolyt...
Text Solution
|