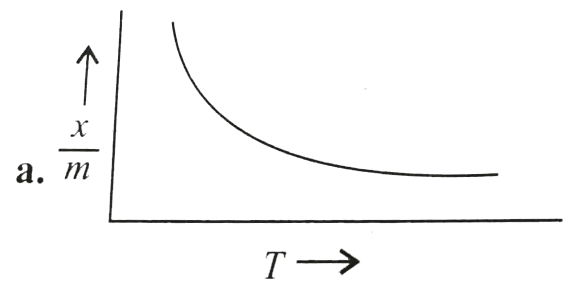
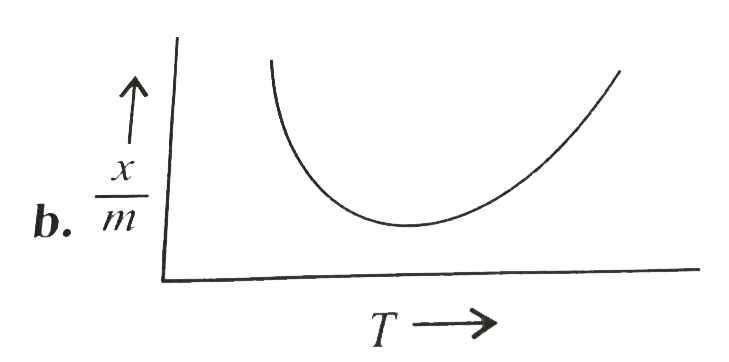
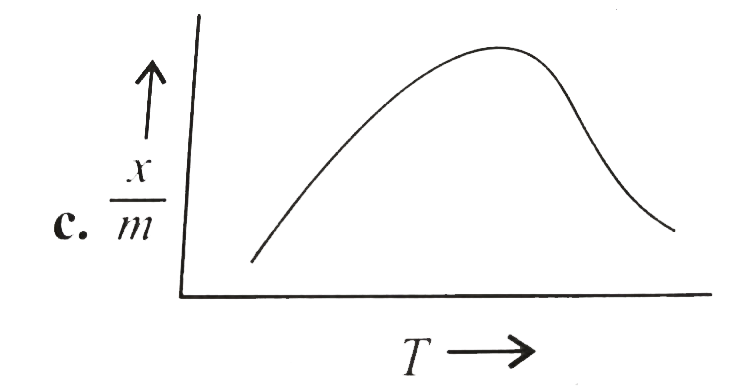
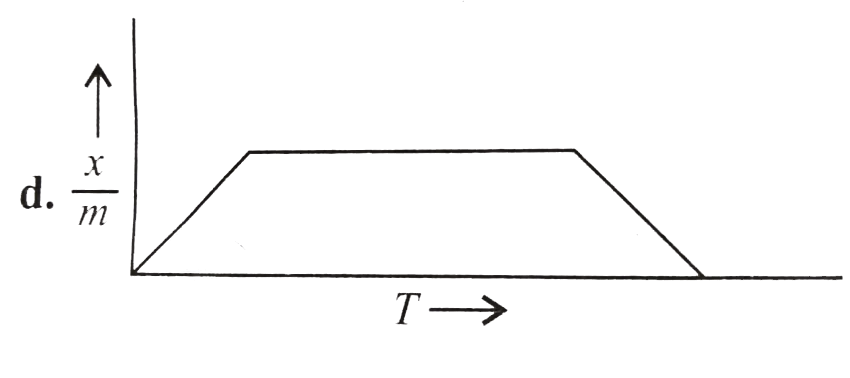
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct|52 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Single Correct Answer Type|2 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Link Comprehension|37 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 2.3 (Objective)|9 VideosSYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion Reasoning|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-SURFACE CHEMISTRY-Exercises Multiple Correct
- Which of the following is/are aerosols?
Text Solution
|
- Which of the following increase(s) the activation of a solid adsorbent...
Text Solution
|
- Which of the following is/are lyphobic colloids?
Text Solution
|
- Which of the following statements is/are correct ?
Text Solution
|
- Which of the following is/are not correctly matched?
Text Solution
|
- Which one of the following is/are correct statement for physisorption
Text Solution
|
- Which of the following statements is/are correct ?
Text Solution
|
- Which of the following electrolytes will not be most effective in the ...
Text Solution
|
- Which of the following are macromolecular colloids?
Text Solution
|
- Isoelectric point is the pH at which colloidal particles
Text Solution
|
- Tyndall effect is applicable when
Text Solution
|
- Multimolecular colloids are present in?
Text Solution
|
- Which of the following belong(s) to the family of enzymes?
Text Solution
|
- Which of the following is/are not possible in case of autocatalysis?
Text Solution
|
- Which is not adsorption isobar for chemisorption?
Text Solution
|
- Which of the following is/are the characteristic of a catalyst?
Text Solution
|
- Which one of the followings is/are an example of homogeneous catalysis...
Text Solution
|
- Efficiency of the catalyst does not depend on its?
Text Solution
|
- Which of the following is/are application(s) of adsorption?
Text Solution
|
- Which of the following statements is/are correct in the case of hetero...
Text Solution
|