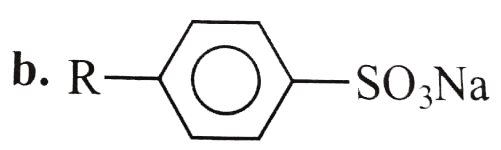
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
SURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Single Correct Answer Type|2 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion-Reasoning|17 VideosSURFACE CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|25 VideosSOLUTIONS
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 2.3 (Objective)|9 VideosSYNTHETIC AND NATURAL POLYMERS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assertion Reasoning|10 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-SURFACE CHEMISTRY-Exercises Single Correct
- Amount of gas adsorbed per gram of adsorbent increases with pressure, ...
Text Solution
|
- Softening of hard water is done using sodium aluminium silicate (zeoli...
Text Solution
|
- Anionic surfactants are
Text Solution
|
- What are surfactants?
Text Solution
|
- What are surfactants?
Text Solution
|
- Micelles are
Text Solution
|
- Compared to common colloidal sols milcells have:
Text Solution
|
- Which one of the following statements is not correct?
Text Solution
|
- Catalyst increases the rate by
Text Solution
|
- the catalyst used in the hydrogenation of oils is :
Text Solution
|
- Which of the following is present at the time of cracking of hydrocarb...
Text Solution
|
- Which is not the correct statement for a catalyst?
Text Solution
|
- Catalyst used in polymerization of ethen is:
Text Solution
|
- Gold number of a lyophic sol is such a property that
Text Solution
|
- Energy of activation of forward and backward reaction are equal in cas...
Text Solution
|
- Which one of the following is a nature colloid?
Text Solution
|
- Collidal solutios of gold prepared by different methods are of differe...
Text Solution
|
- Bleeding is stopped by the application of ferric chloride. This is bec...
Text Solution
|
- If liquid is dispersed in solid medium, then this is called as:
Text Solution
|
- Freundlich equation for adsorption of gases (in amount of Xg) on a sol...
Text Solution
|