Text Solution
Verified by Experts
Topper's Solved these Questions
NCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type Questions|69 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise NCERT Exercise|104 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Nuclear Chemistry (NCERT Exercise)|29 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Linked Comprehension)|1 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|13 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-NCERT BASED EXERCISE-NCERT Exerise
- How will you distinguish between the following pairs of terms: (i) H...
Text Solution
|
- How many lattice points are there in one unit cell of each of the foll...
Text Solution
|
- Explain (i) The basis of similaritites and differences between metal...
Text Solution
|
- What is the efficiency of packing in case of a metal crystal for (i)...
Text Solution
|
- Silver crystallises in fcc lattice. If edge length of the cell is 4.07...
Text Solution
|
- A cubic solid is made of two elements P and Q. Atoms of Q are at the c...
Text Solution
|
- Niobium crystallises in body-centred cubic structure if density is 8.5...
Text Solution
|
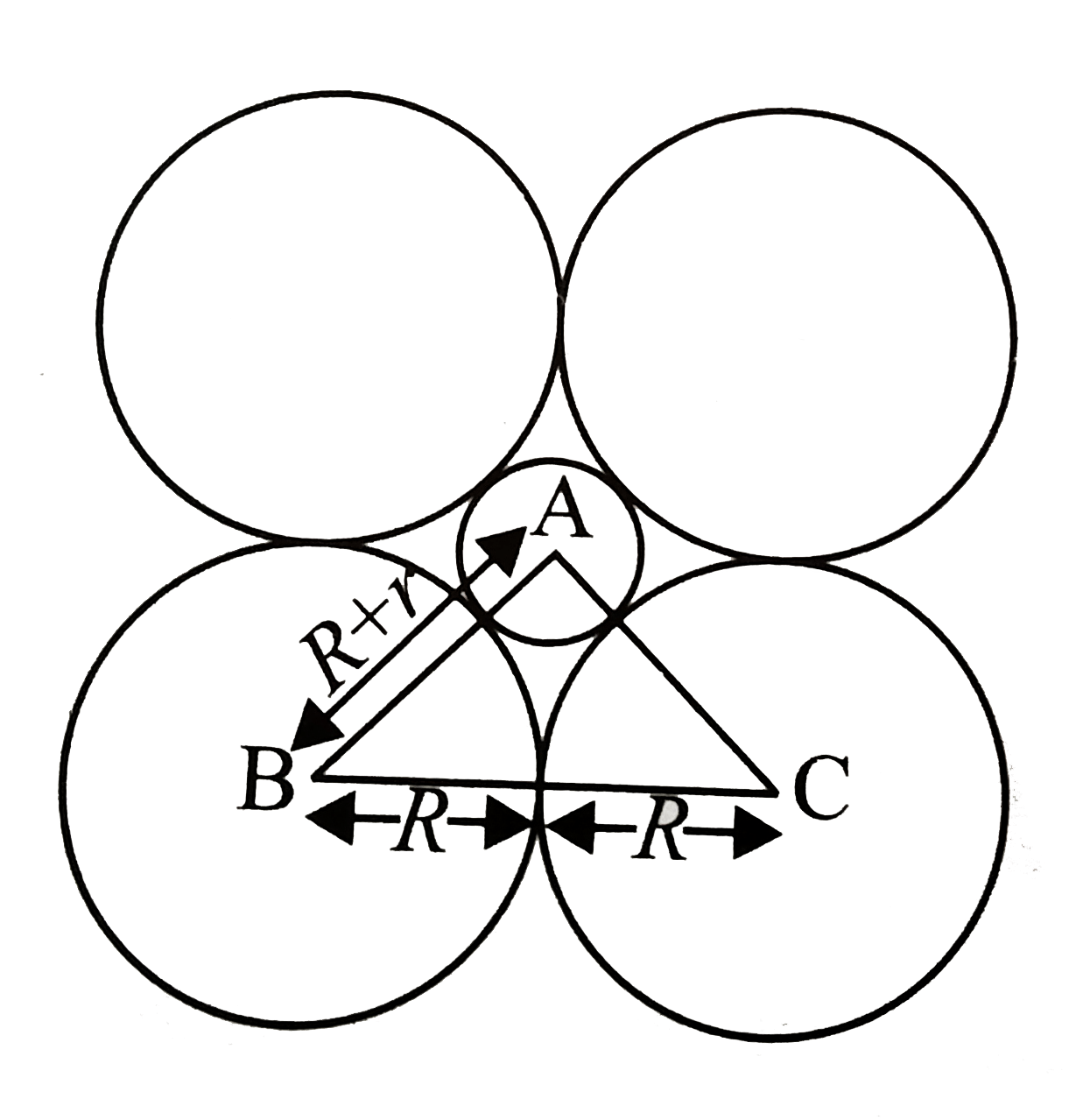
- If the radius of an octahedral void is r and radius of atoms in close...
Text Solution
|
- Copper crystallizer into an fcc lattice with edge length 3.61 xx 10^(-...
Text Solution
|
- Analysis shows that nickel oxide has the formula Ni(0.00). What fracti...
Text Solution
|
- What is a semiconductor? Describe the two main types of semiconductors...
Text Solution
|
- Non-stoichiometric cuprous oxide, Cu(2)O can be prepared in lboratory....
Text Solution
|
- Ferric oxide crystallises in a hexagonal close-packed array of oxide i...
Text Solution
|
- Classify each of the following as being either a p-type of a n-type s...
Text Solution
|
- Gold (atomic radius=0.144nm( crystallises in a face-centred unit cell....
Text Solution
|
- In terms of band theory, what is the difference (i) between a conduc...
Text Solution
|
- Explain the following terms with suitable example: a. Schottky defec...
Text Solution
|
- Aluminium crystallises in a cubic close-packed structuure. Its metalli...
Text Solution
|
- If NaCl is doped with 10^(-4)"mol"% of srCl(2), the concentration of c...
Text Solution
|
- Explain each of the following with a suitable example: Paramagnetism .
Text Solution
|