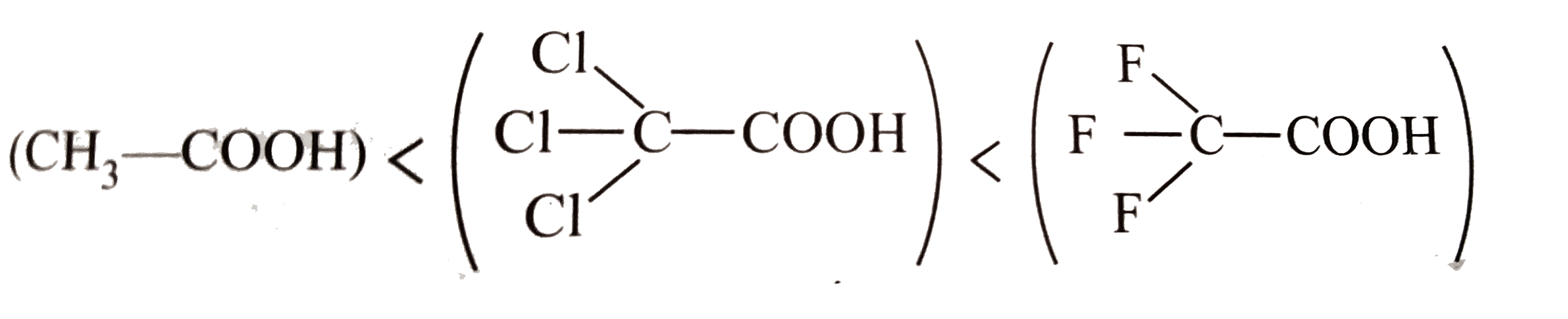Text Solution
Verified by Experts
Topper's Solved these Questions
NCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type Questions(ElectroCHMmical Cell)|4 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type Questions(Electrochmical Cell)|12 VideosNCERT BASED EXERCISE
CENGAGE CHEMISTRY ENGLISH|Exercise Short Answer Type Questions|69 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Linked Comprehension)|1 VideosNUCLEAR CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|13 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-NCERT BASED EXERCISE-NCERT Exercise
- Nalorphene (C(19)H(21)ONO(3)), similar to morphine, is used to combat ...
Text Solution
|
- Calculate the amound of benzoic acid (C(6)H(5)COOH) required for prepa...
Text Solution
|
- The depression in freezing point of water observed for the same amount...
Text Solution
|
- Calculate the depression in the freezing point of water when 10g of CH...
Text Solution
|
- 19.5g of CHM(2)FCOOH is dissolved in 500g of water . The depression i...
Text Solution
|
- Vapour pressure of water at 293K is 17.535 mm Hg. Calculate the vapour...
Text Solution
|
- Henry's law constant for molality of methane in benzene at 298 K is 4....
Text Solution
|
- 100g of liquid A (molar mass 140g "mol"^(-1)) was dissolved in 1000g o...
Text Solution
|
- The heat of formation of ethane is -19.46 kcal. Bond enegries of H-H, ...
Text Solution
|
- The air is a mixture of a number of gases. The maojr components are ox...
Text Solution
|
- Determine the amount of CaCl(2) (i = 2.47) dissolved in 2.5 L of water...
Text Solution
|
- Determine the osmotic pressure of a solution prepared by dissolving 25...
Text Solution
|
- Arrange the following metals in the order in which they displace each ...
Text Solution
|
- Given the standard electrode potentials , K^(+)//K = - 2.93 V , Ag^(...
Text Solution
|
- Depict the galvanic cell in which the reaction Zn(s) + 2 Ag^(+) (aq)...
Text Solution
|
- What will be standard cell potential of galvanic cell with the followi...
Text Solution
|
- Write the nernst equation and emf of the following cells at 298 K: (...
Text Solution
|
- In the button cells widely used in watches and other devices the follo...
Text Solution
|
- Definie conductivity and molar conductivity for the solution of an ele...
Text Solution
|
- The conductivity of 0.20 M solution of KCl at 298 K is 0.0248S" "cm^(-...
Text Solution
|