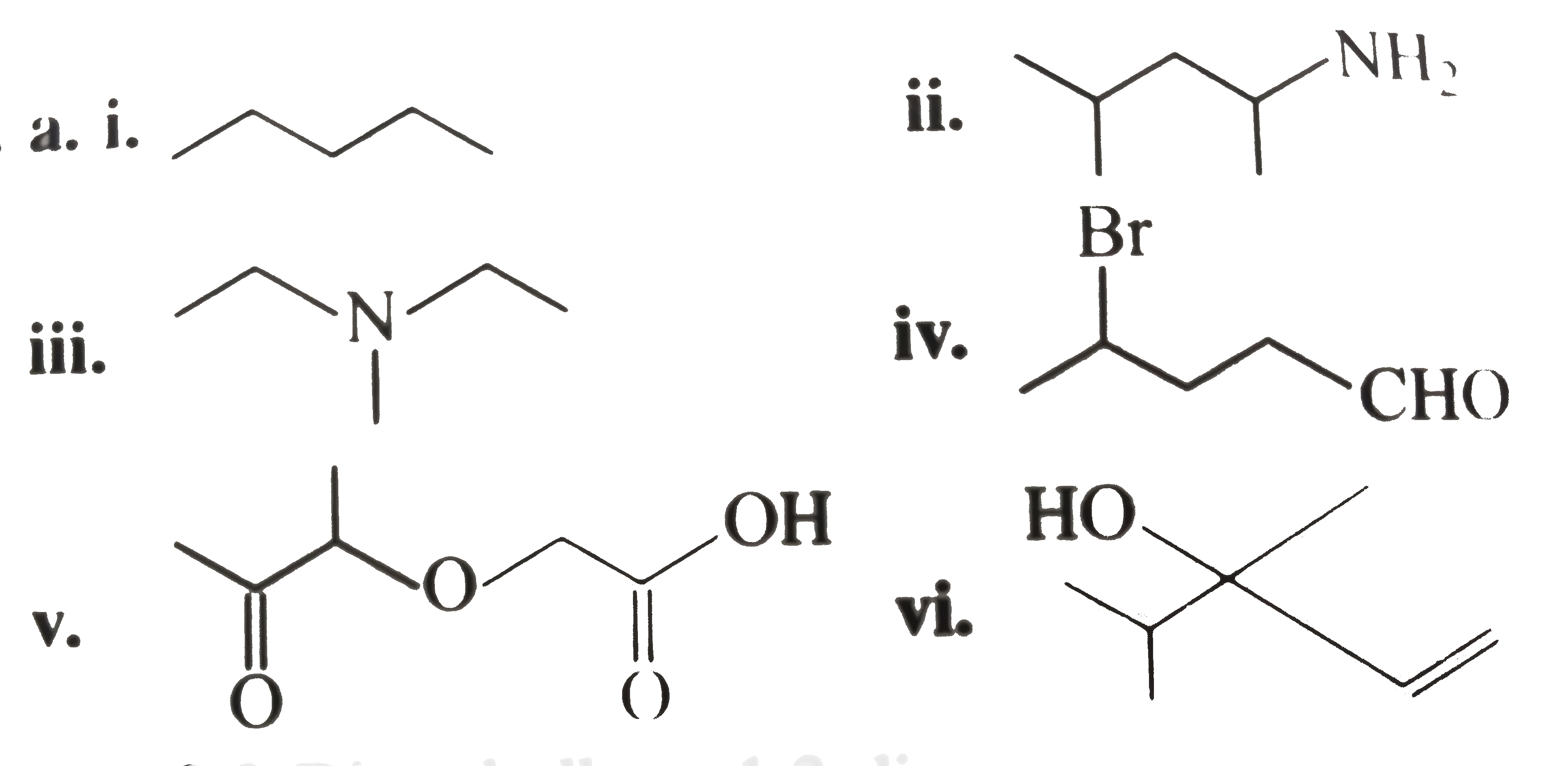
Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Solved Example|5 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises|10 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective type|1 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive|1 Videos
Similar Questions
Explore conceptually related problems