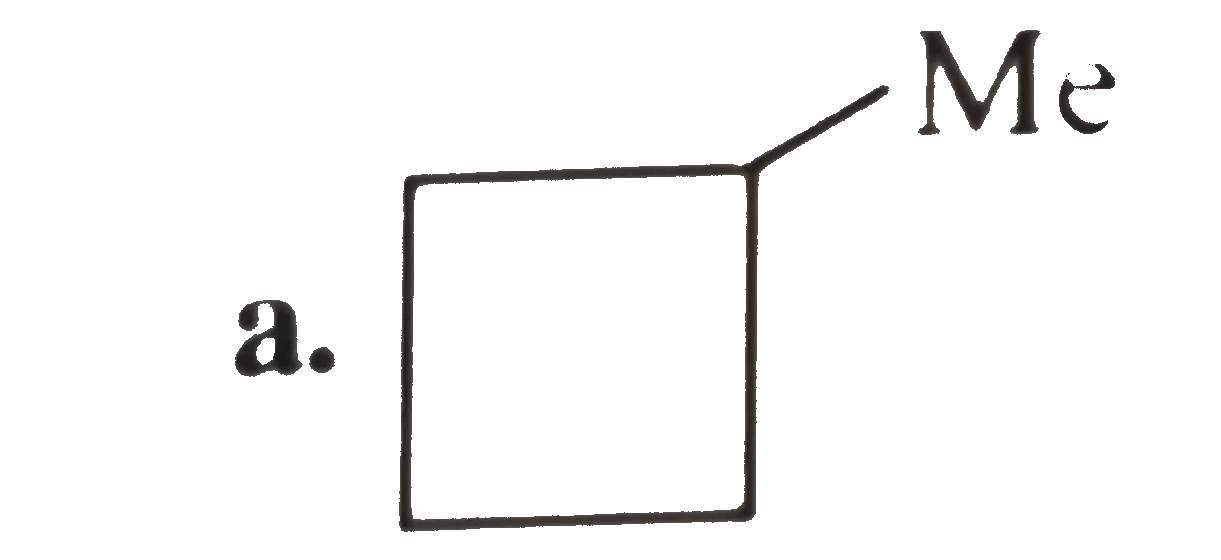
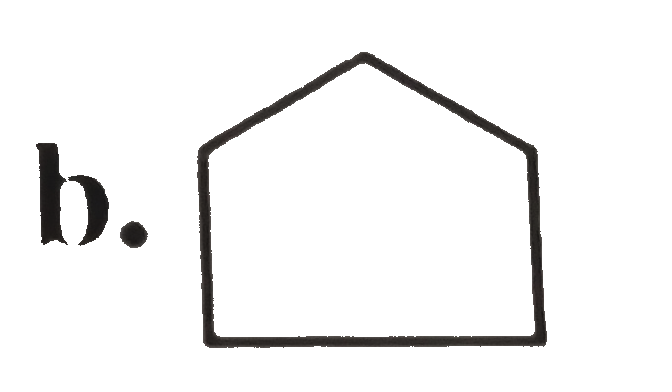
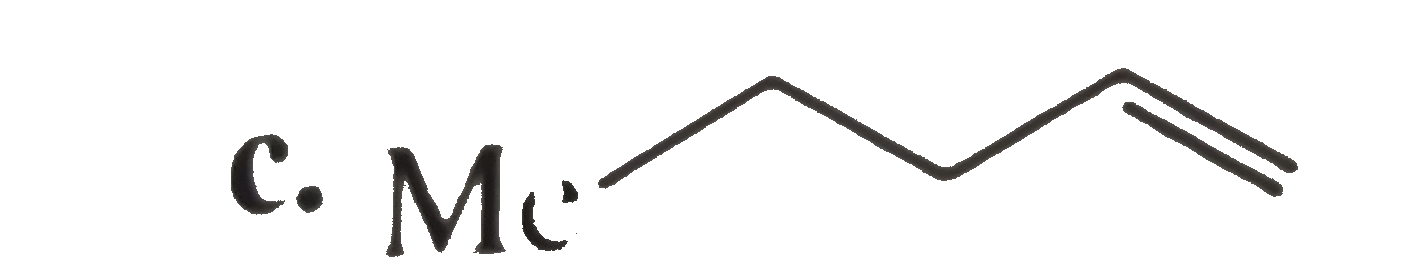
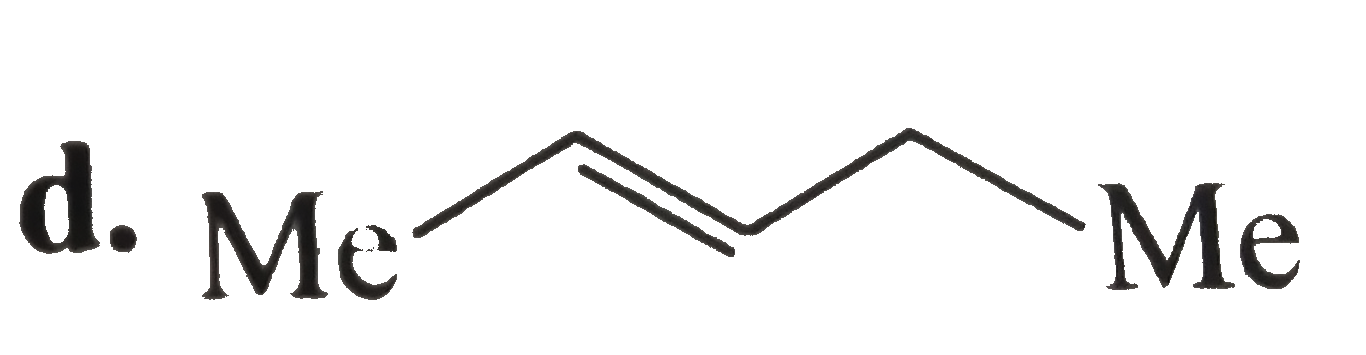
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion-Reasoning Type|5 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Fill in the Blanks Type|5 VideosCLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
CENGAGE CHEMISTRY ENGLISH|Exercise Multiple corrcct Answers Types|11 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective type|1 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS-Single correct Answer Type
- The systematic nomencalutre of the following spiro-compound is:
Text Solution
|
- An alkane (A) having a molecular mass of 72 produces one monochlorinat...
Text Solution
|
- A compound (A) with molecular formula C(5) H(10) gives one monochlorin...
Text Solution
|
- Which of the following the following in a 3^(@) amine?
Text Solution
|
- Which of the following as a 3^(@) alcohol?
Text Solution
|
- Which of the following is zerone?
Text Solution
|
- Which of the following is pyrogallol?
Text Solution
|
- In 3-chloro cyclohexanol, the primary prefix is:
Text Solution
|
- In 2-Chloro-3-methy1 hexanoic acid, the primary suffix is:
Text Solution
|
- The correct name of the compound (I) is:
Text Solution
|
- Which of the following is not a cumulated diene?
Text Solution
|
- Which of the following has only 1^(@) and 2^(@)C atoms?
Text Solution
|
- The IUPAC name of viny1 acetylene is:
Text Solution
|
- Which of the following structures represents cyclopenty1 methy1 carbin...
Text Solution
|
- The IUPAC name of acrolein is:
Text Solution
|
- The IUPAC name of the following compound is
Text Solution
|
- Which of the following is not the name of CH(3) NC?
Text Solution
|
- The IUPAC name of Ph-CN is :
Text Solution
|
- Give the IUPAC name of :
Text Solution
|
- Which of the following statements is wrong for homologous series?
Text Solution
|