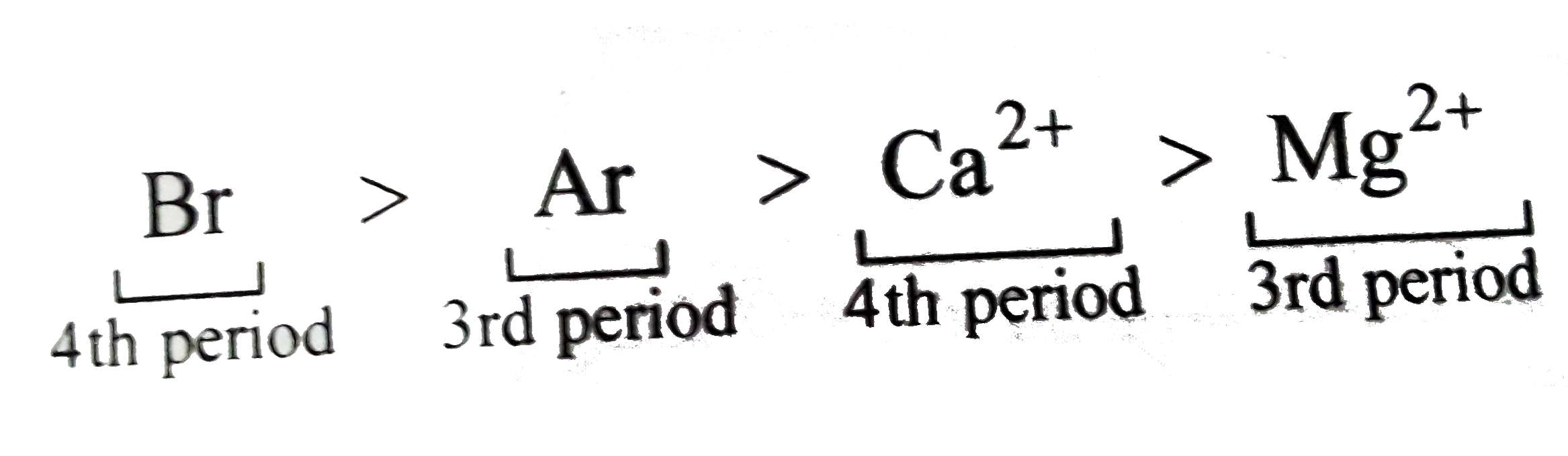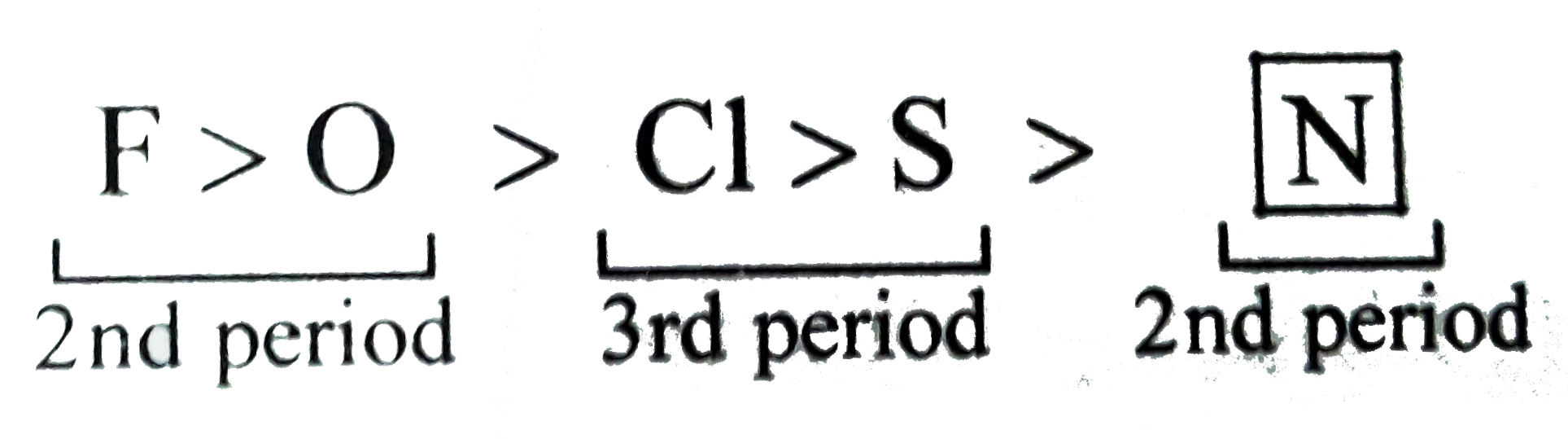Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.1|14 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 1.1 (Very Short)|22 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Archives )Subjective|4 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|9 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion Reasoning Type|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY-Solved Examples
- Identify the three elements A, B and C from the data given below a. ...
Text Solution
|
- Among the elements, Ar, Si, Na and Cl. Select an elements with a. Hi...
Text Solution
|
- Arrange in decreasing order as directed. (a) Decreasing order of EN:...
Text Solution
|
- Predict from each set, the atom/ion which has the greatest IE(1) with ...
Text Solution
|
- The Delta(eg)H^(ɵ) of Br is 3.4 eV. How much energy in kcal is release...
Text Solution
|
- Predict from each set, the element which has the more negative electro...
Text Solution
|
- The electronegativity of caesium is 0.7 and that of fluorine is 4.0 . ...
Text Solution
|
- Among the elements with Z = 9, 12 and 36, identify by atomic number of...
Text Solution
|
- Explain the question (based on ionisation energy): (a) Why IE(1) of ...
Text Solution
|
- Answer the following question (Based on EA, Delta(eg)H^(ɵ) and IE). I...
Text Solution
|
- a. Arrange the following species in decreasing order of their sizes//i...
Text Solution
|
- For the gaseous reaction K+F rarrK^(o+)+F^(ɵ) Delta H = 19 kcal mo...
Text Solution
|
- For the gaseous reaction K+F rarrK^(o+)+F^(ɵ) Delta H = 19 kcal mo...
Text Solution
|
- From N atoms of an element A, when half the atoms transfer one electro...
Text Solution
|
- The conservation of gaseous atoms K and F to K^(o+) and F^(ɵ) absorbs ...
Text Solution
|
- Which of the following Na, Mg, Si and P would have the greatest differ...
Text Solution
|
- Classify the following oxides as a. Strongly acidic b. Weakly acidic...
Text Solution
|
- Select the strongest and weakest acid in each of the following sets: ...
Text Solution
|
- A 0.10 M aqueous solution of which salt in the following pair would ha...
Text Solution
|
- Identify: a. The good oxidising agent b. The good reducing agent ...
Text Solution
|