A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correct) Isoelectronic Species|4 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Single Correct) Matallic-Non Metallic Character|3 VideosPERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Singlecorrect) Atomic And Ionic Radii|15 VideosP-BLOCK GROUP 14 - CARBON FAMILY
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Subjective)|9 VideosPURIFICATION OF ORGANIC COMPOUNDS AND QUALITATIVE AND QUANTITATIVE ANALYSIS
CENGAGE CHEMISTRY ENGLISH|Exercise Assertion Reasoning Type|5 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-PERIODIC CLASSIFICATION OF ELEMENTS AND GENERAL INORGANIC CHEMISTRY-Exercises (Single Correct) Ionisation Energy (Ie)
- Which of the following process refers to IE(2) ?
Text Solution
|
- Which of the following statement concerning ionisation energy is not c...
Text Solution
|
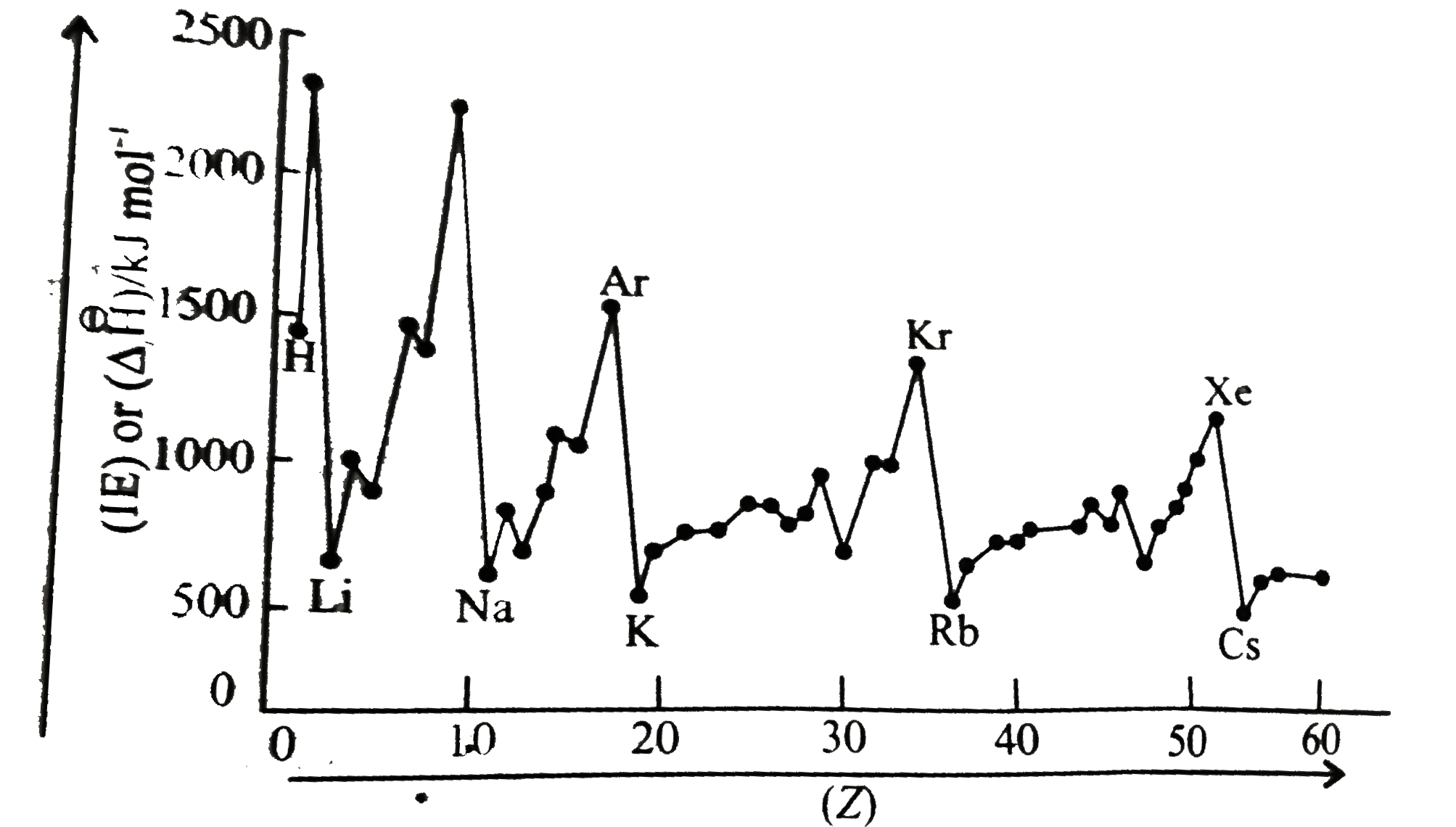
- The graph of IE(1) or Delta(1)H(1)^(ɵ) versus atomic number (Z) is giv...
Text Solution
|
- Which of the following ioelectronic ions have the lowest ionisation en...
Text Solution
|
- The second ionisation potential is
Text Solution
|
- Which of the following process requires the largest amount of energy ?
Text Solution
|
- Which of the following in an energy consuming process ?
Text Solution
|
- Arrange S, P and As in order of increasing ionisation energy.
Text Solution
|
- The five successive ionization enthalpies of an element are 800, 2427 ...
Text Solution
|
- Which of the following transitions involves maximum amount of energy?
Text Solution
|
- Which of the elements show least values of ionisation within their per...
Text Solution
|
- Which of the following has the largest ionisation energy.
Text Solution
|
- Which one of the following elements has the highest ionisation energy?
Text Solution
|
- The correct order of second ionisation potentials of carbon, nitrogen,...
Text Solution
|
- Which has the largest first ionisation energy ?
Text Solution
|
- Which of the following element has the highest ionisation enregy ?
Text Solution
|
- Ionisation enthalpy of nitrogen is more than oxygen because of
Text Solution
|
- The set representing the correct order of the first ionisation potenti...
Text Solution
|
- The first ionisation potential of which of the element is highest
Text Solution
|
- Highest ionisation potential in a period is shown by
Text Solution
|