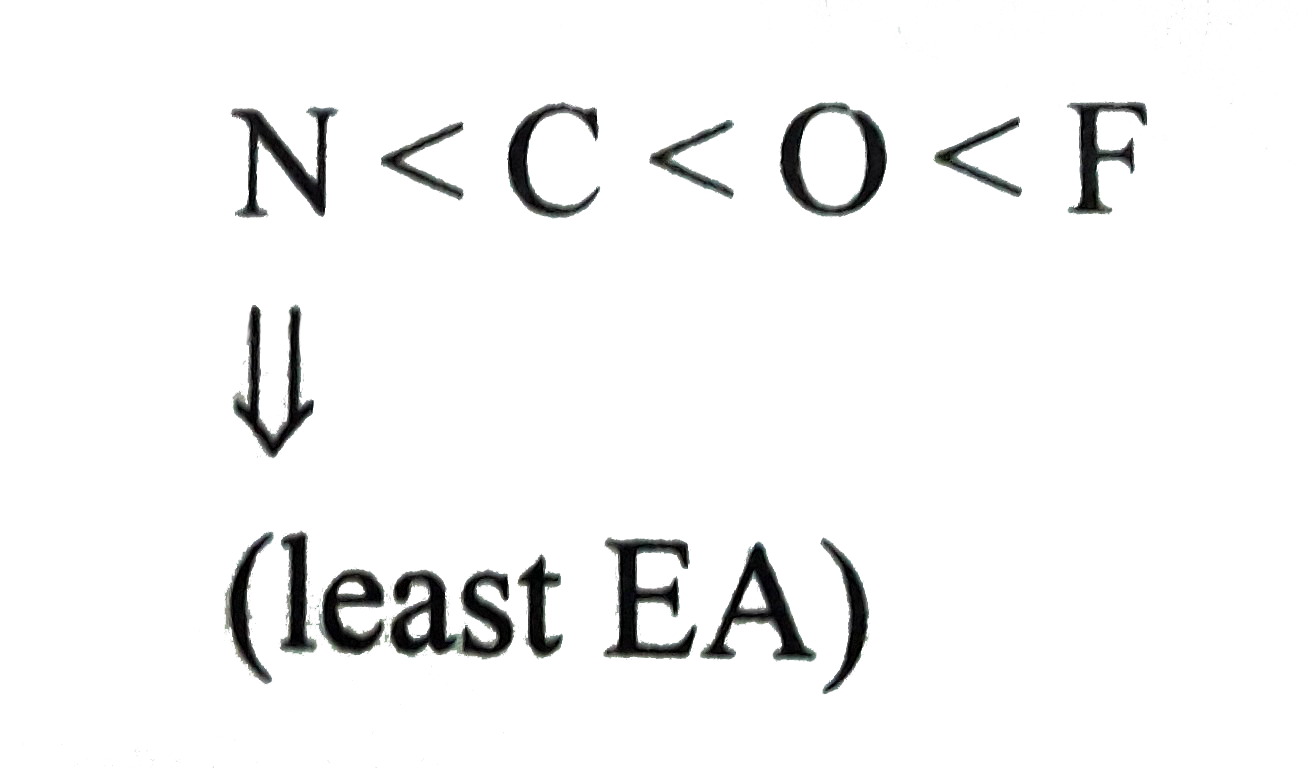A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Which of the following will ave lowest electron affinity?
Text Solution
|
- Among (the non-radioactive) halogens the element that has the lowest e...
Text Solution
|
- Electron affinity is the lowest for
Text Solution
|
- Which of the following elements has zero electron affinity ?
Text Solution
|
- The one with lowest electron affinity in group 16 is
Text Solution
|
- Which one possesses lowest electron affinity ? B , C , N , O
Text Solution
|
- Which of the following represents correct order of electron affinity?
Text Solution
|
- Which of the following will have the lowest electron affinity?
Text Solution
|
- Which of the following is the correct order of electron affinity
Text Solution
|