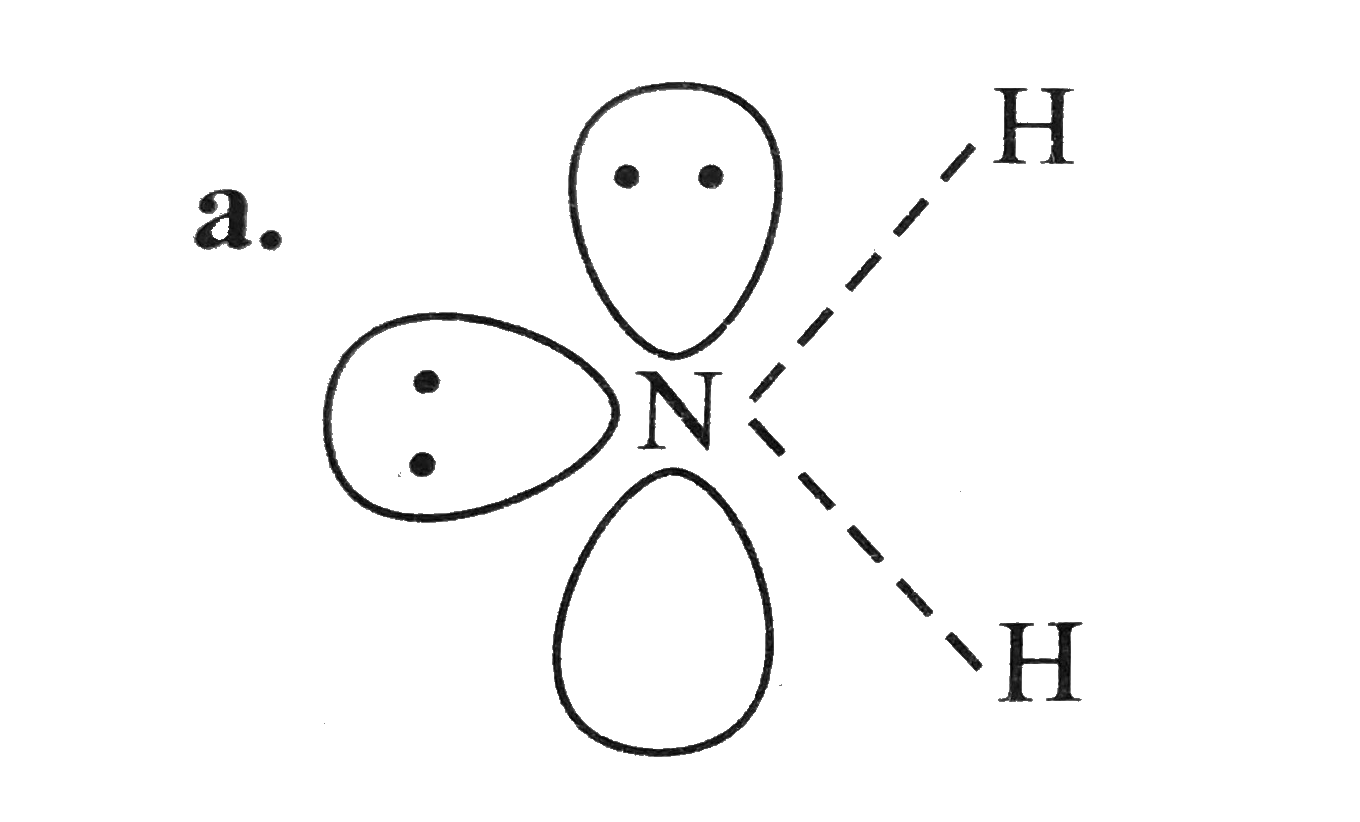
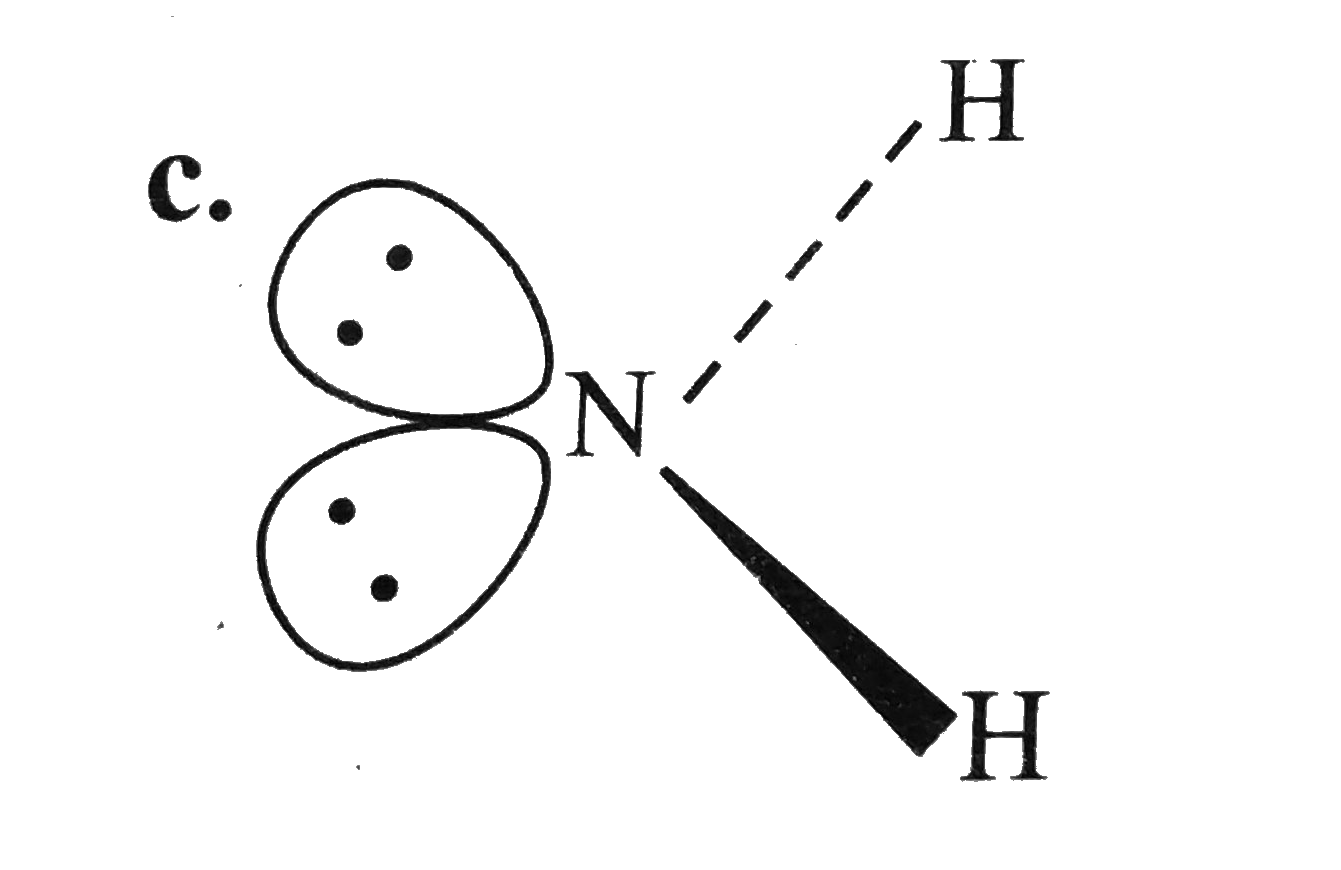
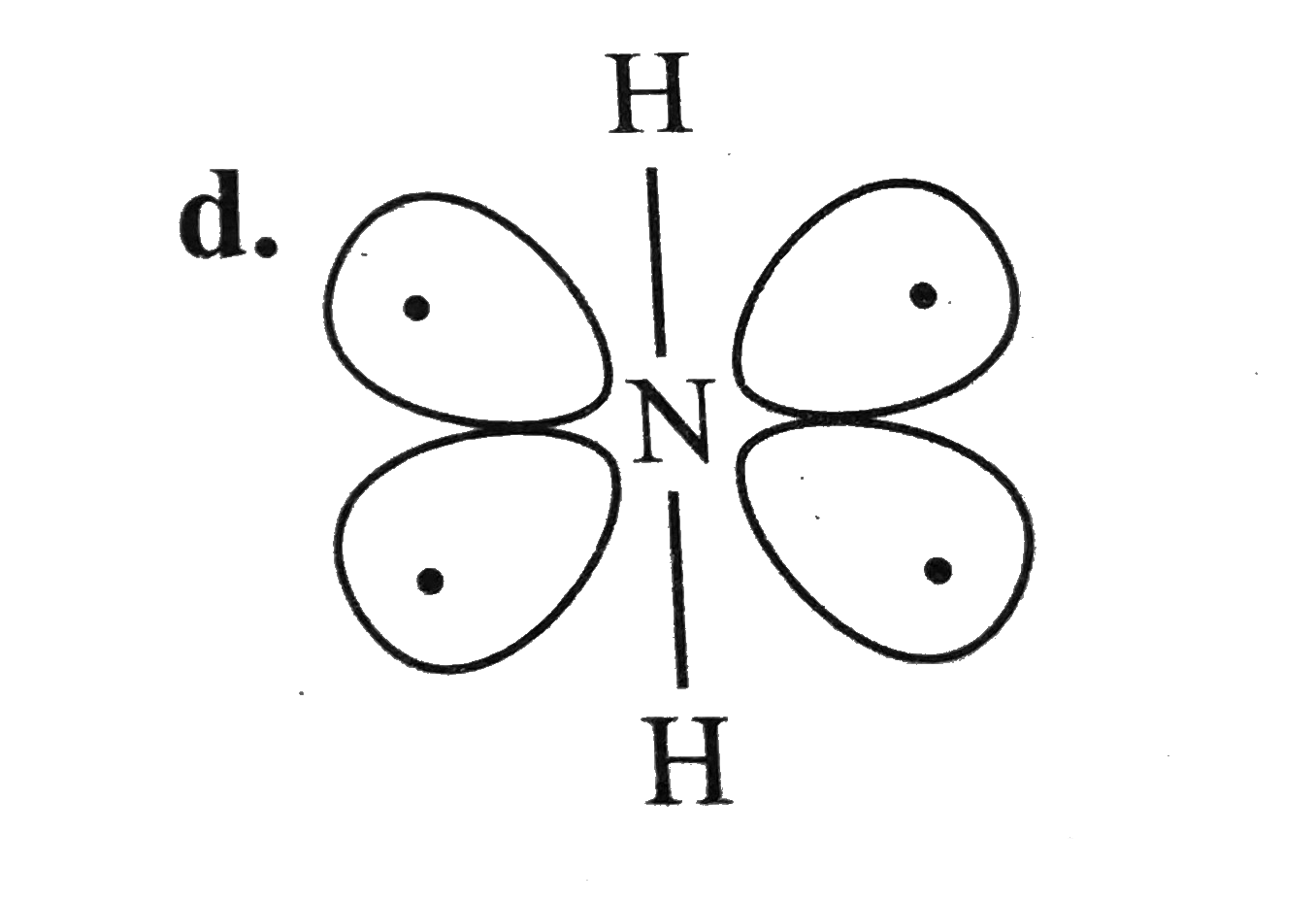
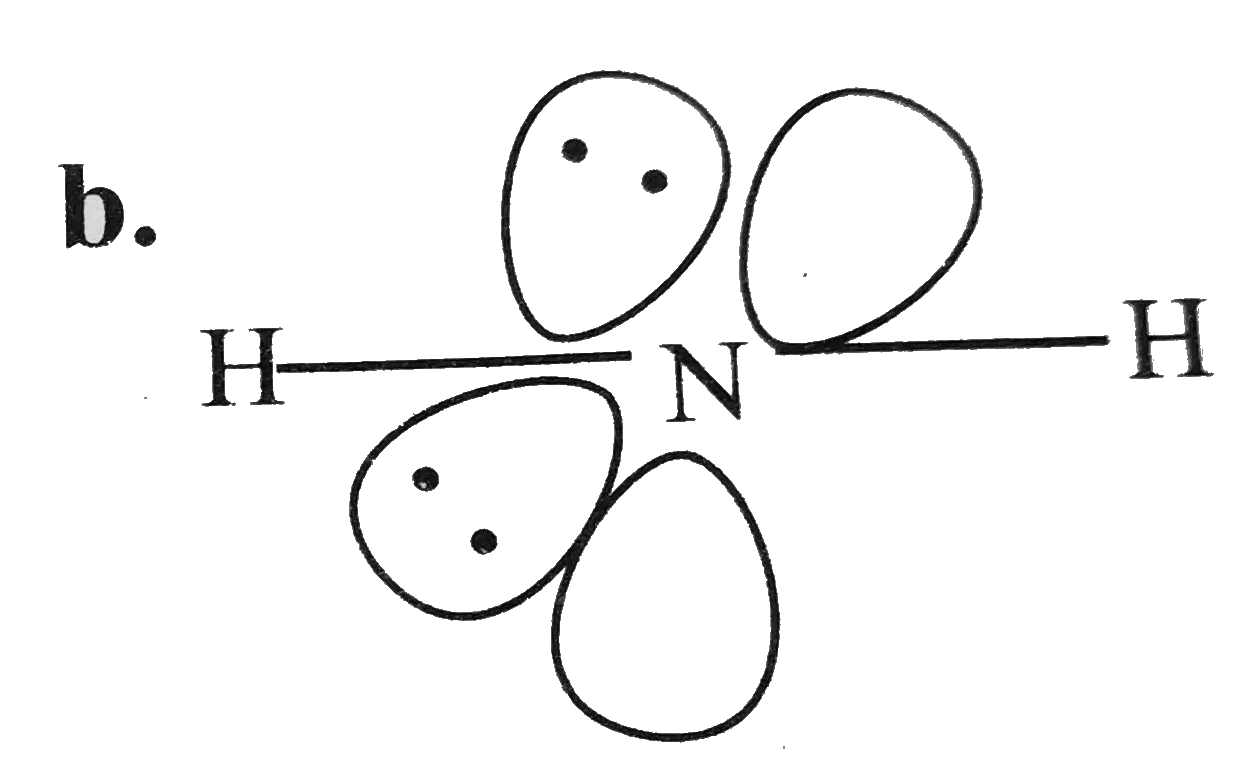A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Assetion Reasoning|30 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Integer|10 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Single Correct (Molecular Orbital Theory (Mot))|23 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Exercises Single Correct (Miscellaneous)
- Which is the wrong order for the stated property ? .
Text Solution
|
- Which is a correct statement about diborane structure ? .
Text Solution
|
- For overset(Theta)NH(2) the best three-dimensional view is .
Text Solution
|
- The set representing the correct order of ionic radius is
Text Solution
|
- Which of the following are not isoelectronic speices ?
Text Solution
|
- The EN's of F, CI Br and I are 4.0 ,3.0, 2.8 and 2.5 respectively The ...
Text Solution
|
- The C -C bond length is 1.54 Å C =C bond length is 1.33 Å What is the ...
Text Solution
|
- The correct order of the thermal stability of hydrogen halides (H-X) i...
Text Solution
|
- Which of following statement is correct ? .
Text Solution
|
- The valuse of EN of atoms A and B are 1.80 and 4.0 respectively The pe...
Text Solution
|
- The statement true for N(3)^(-) is
Text Solution
|
- The decreasing (O -O) bonf length order in the following is .
Text Solution
|
- Which of the following substance has the highest melting point? .
Text Solution
|
- Which of the following statement is correct ? .
Text Solution
|
- Which of the following bonds is the strongest ? .
Text Solution
|
- The molecule having highest bond enegy is
Text Solution
|
- Which set is expected to show the smallest difference in IE(1) ?
Text Solution
|
- Which of the following statement is wrong ? .
Text Solution
|
- Which of the following is correct ?
Text Solution
|
- Lattice energy of BeCO(3)(I), MgCO(3)(II) and CaCO(3)(III) is in order...
Text Solution
|