A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Integer|2 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Fill In The Blanks|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Multiple Correct|10 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Archives Single Correct
- Polar covalent molecules exhibit dipole moment. Dipole moment is equal...
Text Solution
|
- CN^(Theta) and N(2) are isoelectronic But in contrast to CN^(Theta),N(...
Text Solution
|
- Among KO(2), KAlO(2), CaO(2) and NO(2)^(+), unpaired electrons is pres...
Text Solution
|
- Which one of the following compounds has sp^(2) hybridisation ? .
Text Solution
|
- Among the following compounds the one that is polar and has the centra...
Text Solution
|
- Which contains both polar and non-polar bonds ? .
Text Solution
|
- The correct order of decreasing bond lengths of CO,CO(2) and CO(3)^(2-...
Text Solution
|
- The geometry of H2S and its dipole moment are
Text Solution
|
- Molecular shapes of SF(4), CF(4), XeF(4) are
Text Solution
|
- The types of hybrid orbitals of nitrogen in NO(2)^(+), NO(3)^(-) and N...
Text Solution
|
- The correct order of hybridisation of the central atom in the followin...
Text Solution
|
- Which of the following statement is correct among the species CN^(Θ),C...
Text Solution
|
- Specify the coordination geometry around and the hybridisation of N an...
Text Solution
|
- The least stable ion among the following is
Text Solution
|
- Which of the following species has unpaired electrons?
Text Solution
|
- Which of the following are iso-electronic as well as iso-structural ? ...
Text Solution
|
- Which of the following oxoacids of sulpher has -O-O- linkage ?
Text Solution
|
- According to MO theory,
Text Solution
|
- Number of lone pairs (s) in XeOF(4) is/are
Text Solution
|
- Which species has the maximum number of lone pair of electrons on th...
Text Solution
|
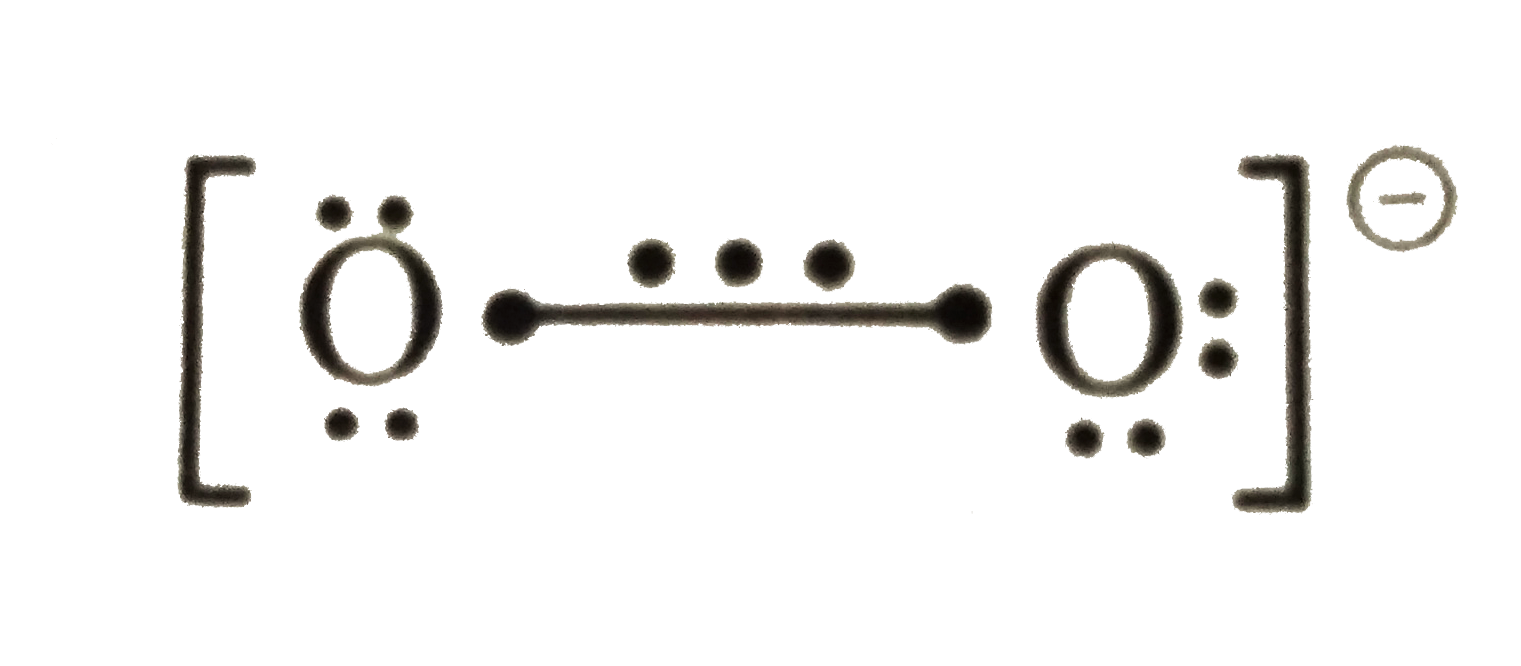 .
.