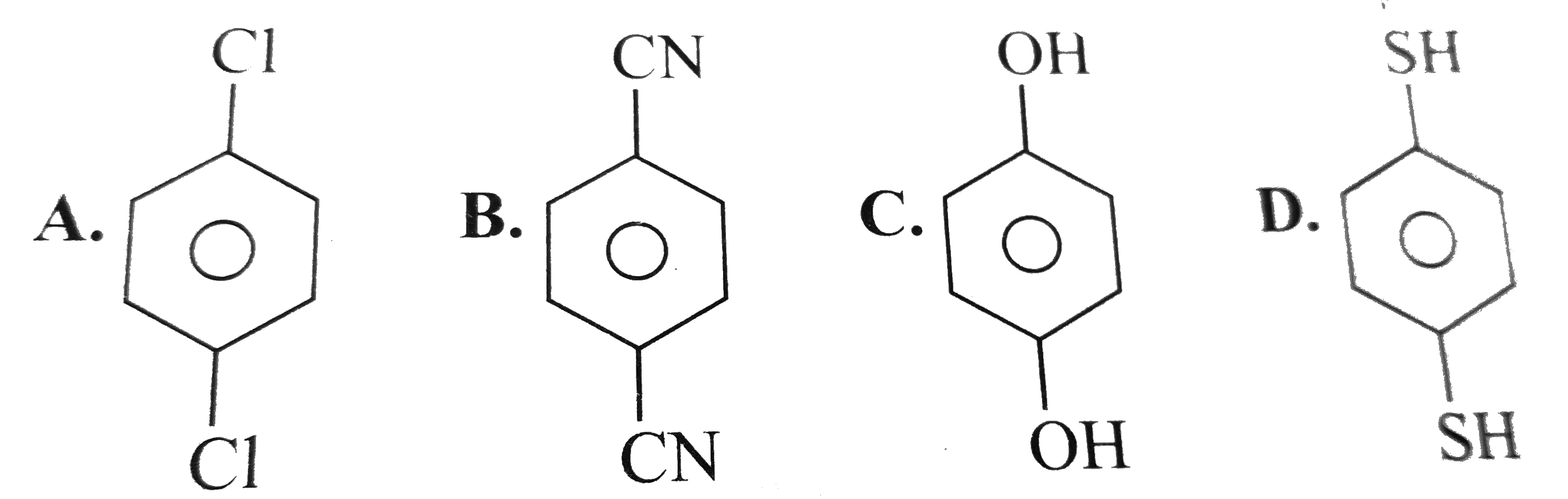
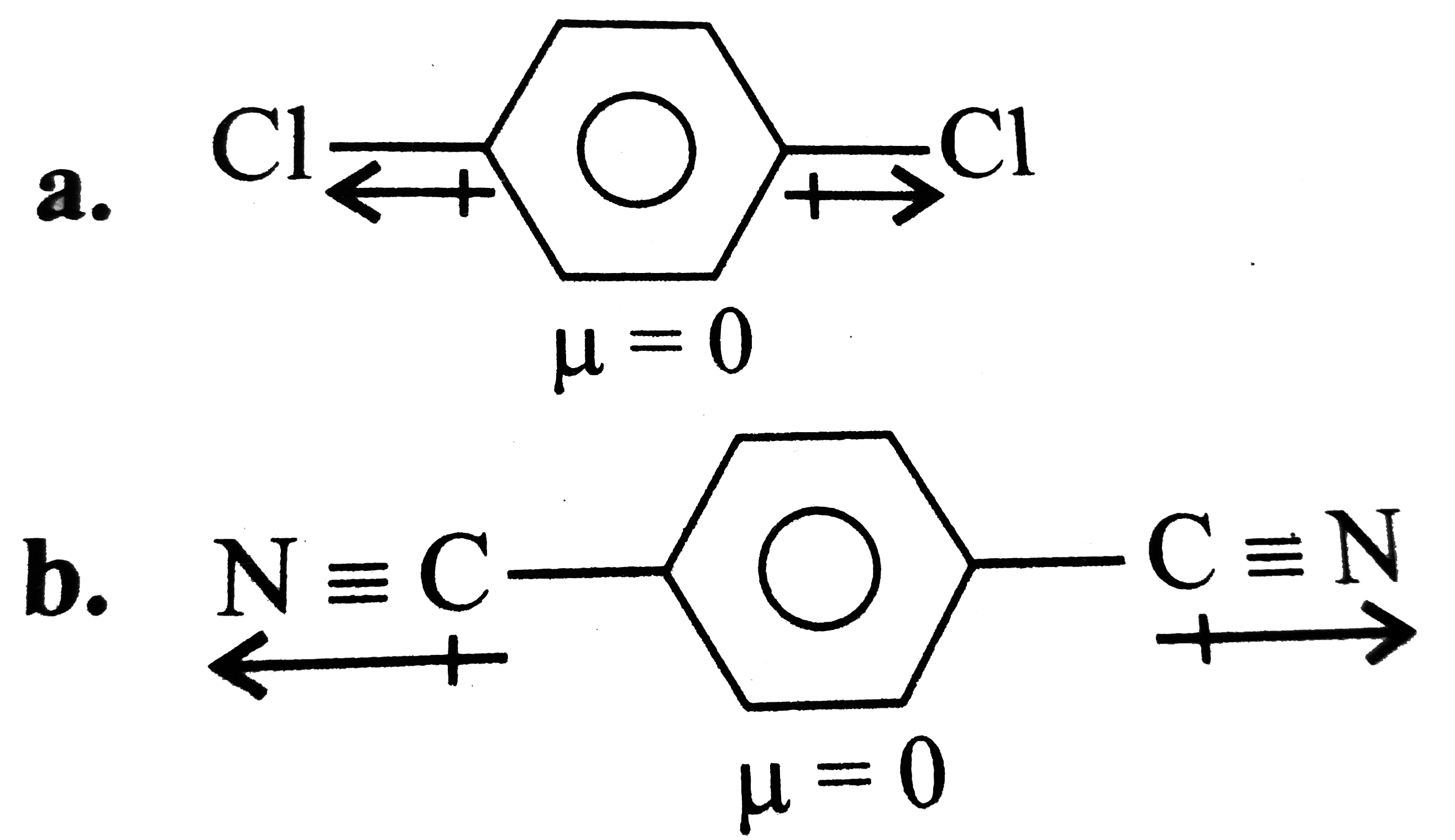
A
B
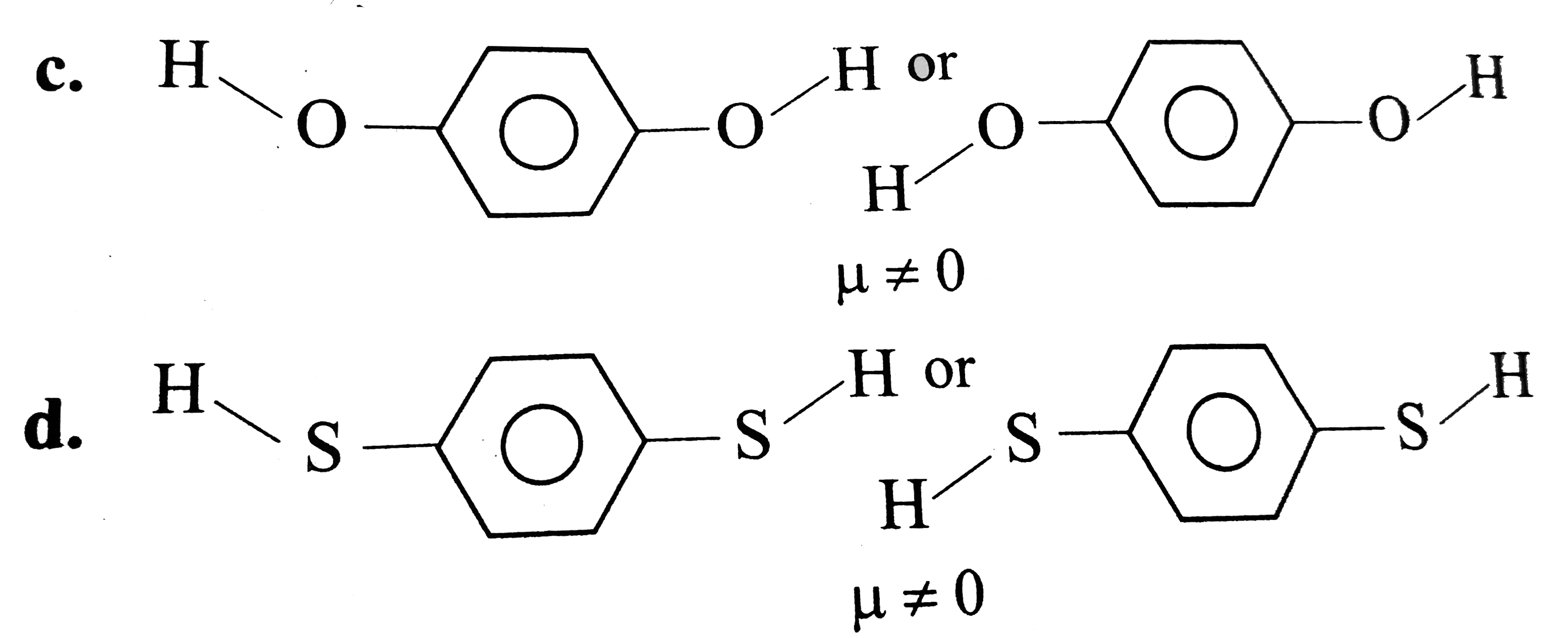
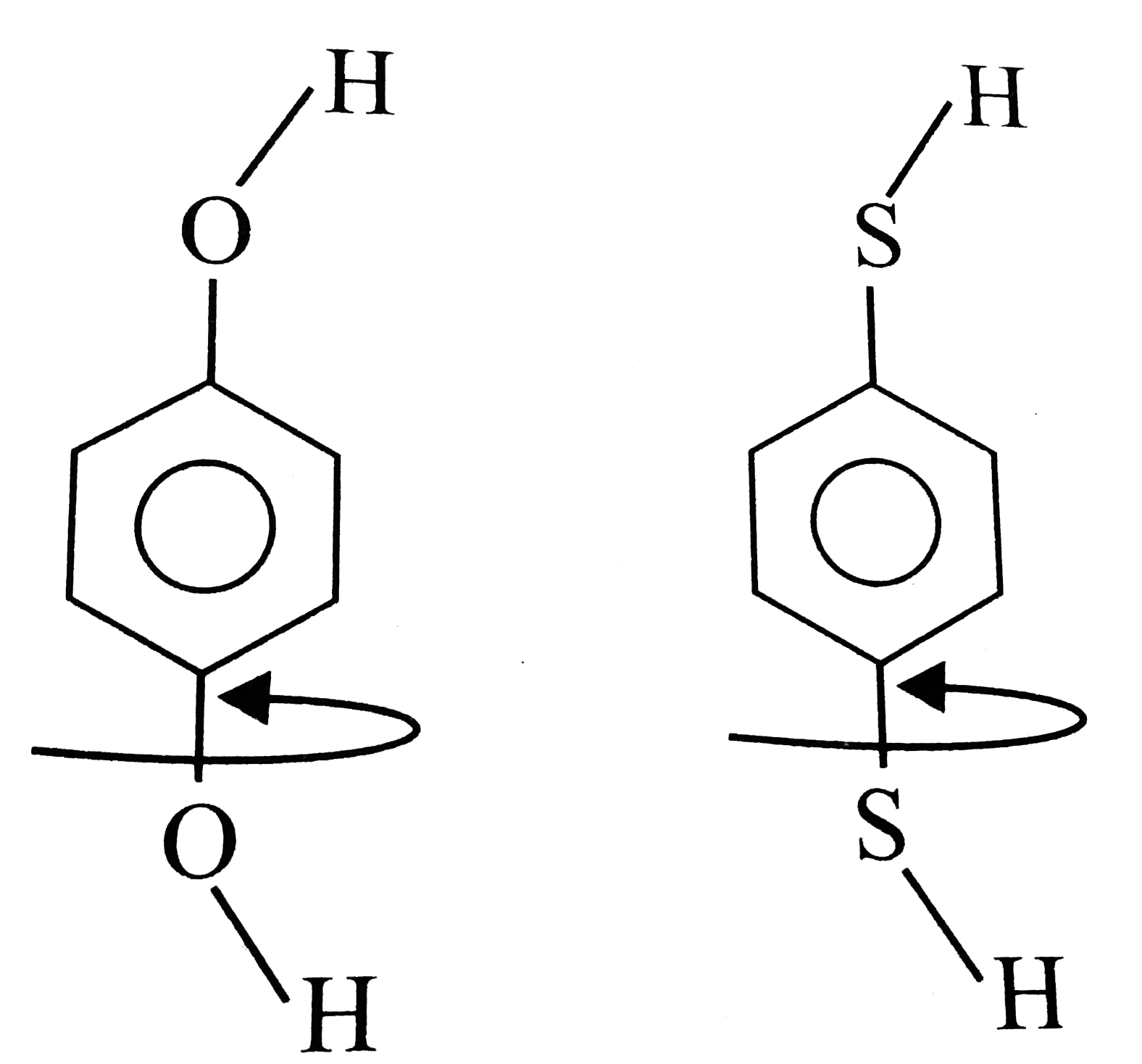
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Integer|2 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Fill In The Blanks|4 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Multiple Correct|10 VideosATOMIC STRUCTURE
CENGAGE CHEMISTRY ENGLISH|Exercise Concept Applicationexercise(4.3)|19 VideosCHEMICAL EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Subjective type|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-CHEMICAL BONDING AND MOLECULAR STRUCTURE-Archives Single Correct
- The correct order of hybridisation of the central atom in the followin...
Text Solution
|
- Which of the following statement is correct among the species CN^(Θ),C...
Text Solution
|
- Specify the coordination geometry around and the hybridisation of N an...
Text Solution
|
- The least stable ion among the following is
Text Solution
|
- Which of the following species has unpaired electrons?
Text Solution
|
- Which of the following are iso-electronic as well as iso-structural ? ...
Text Solution
|
- Which of the following oxoacids of sulpher has -O-O- linkage ?
Text Solution
|
- According to MO theory,
Text Solution
|
- Number of lone pairs (s) in XeOF(4) is/are
Text Solution
|
- Which species has the maximum number of lone pair of electrons on th...
Text Solution
|
- The species having bond order differnet from that in CO is .
Text Solution
|
- Among the following the paramagnetic compound is
Text Solution
|
- The percentage of p-character in the orbitals forming p-p bonds in P4 ...
Text Solution
|
- The molecule which has pyramidal shape is
Text Solution
|
- Which one the following properties is not shown by NO ?
Text Solution
|
- For which of the following molecule significant mu ne0 ?
Text Solution
|
- The correct statement for the molecule, Csl(3) is
Text Solution
|
- Assuming 2s-2p mixing is NOT operative, the paramagnetic species among...
Text Solution
|
- The intermolecular interaction that is dependent on the inverse cube o...
Text Solution
|
- The ionic radii (in Å) of N^(3-), O^(2-) and F^(-) are respectively :
Text Solution
|