Text Solution
Verified by Experts
Topper's Solved these Questions
HYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises (Linked Comprehension )|20 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Multiple Correct|19 VideosHYDROGEN, WATER AND HYDROGEN PEROXIDE
CENGAGE CHEMISTRY ENGLISH|Exercise Ex 3.1|4 VideosGENERAL ORGANIC CHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Analytical and Descriptive|1 VideosIONIC EQUILIBRIUM
CENGAGE CHEMISTRY ENGLISH|Exercise Archives Subjective|28 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-HYDROGEN, WATER AND HYDROGEN PEROXIDE-Ex 3.2
- What is understood by 'Water gas shift reaction' ? Discuss· its use fo...
Text Solution
|
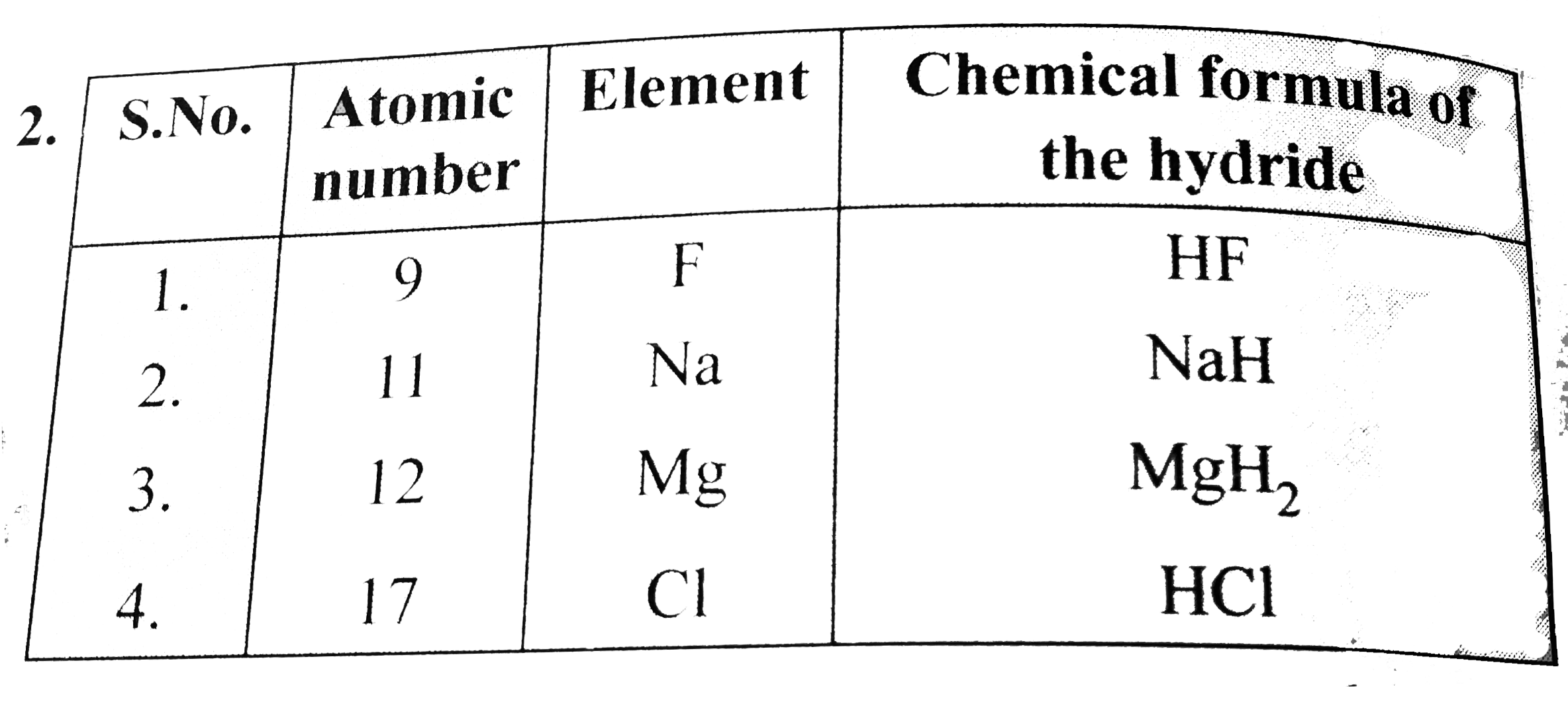
- Hydrogen forms compounds with elements having atomic numbers 9, 11, 12...
Text Solution
|
- What are metallic/interstitial hydrides ? How do they differ form ...
Text Solution
|
- Complete the following reactions : CaO(s)+H(2)O(l)to
Text Solution
|
- Explain, why hydrogen peroxide is stored in coloured/plastic bottles.
Text Solution
|
- Describe the industrial applications of hydrogen which depend on the h...
Text Solution
|
- How would you prepare dihydrogen from water by using a reducing agen...
Text Solution
|
- Complete the following equations : Fe(s)+H(2)O(g)to
Text Solution
|
- Discuss the importance of heavy water in nuclear reactors.
Text Solution
|
- How is heavy water prepared from ordinary water ? Discuss its importan...
Text Solution
|
- Explain, why water has high boiling and melting points as compared to ...
Text Solution
|
- Distinguish clearly between hard and soft water
Text Solution
|
- Explain the correct context in which the following terms are used: ...
Text Solution
|
- Is it correct to say that hydrogen can behave as a metal? If it is cor...
Text Solution
|
- What is importance of the heavier isotopes of hydrogen?
Text Solution
|
- Does hydrogen show allotropy? How many allotropes of dihydrogen are kn...
Text Solution
|
- What is understood by 'hydrogen gap'?
Text Solution
|
- Hydrogen forms three types of bonds in its compounds. Giving suitable ...
Text Solution
|
- Elements with atomic numbers 17 and 20 form compounds with hydrogen. W...
Text Solution
|
- Complete the following reactions. a.CaO((s))+H(2(g))to,b.CO(g)+H(2(g...
Text Solution
|