A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exs 1.1 (Subjective)|25 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exs 1.1 (Objective)|14 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Archives (Subjective)|14 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archieves Subjective|35 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Linked Comprehension)|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS-Solved Example
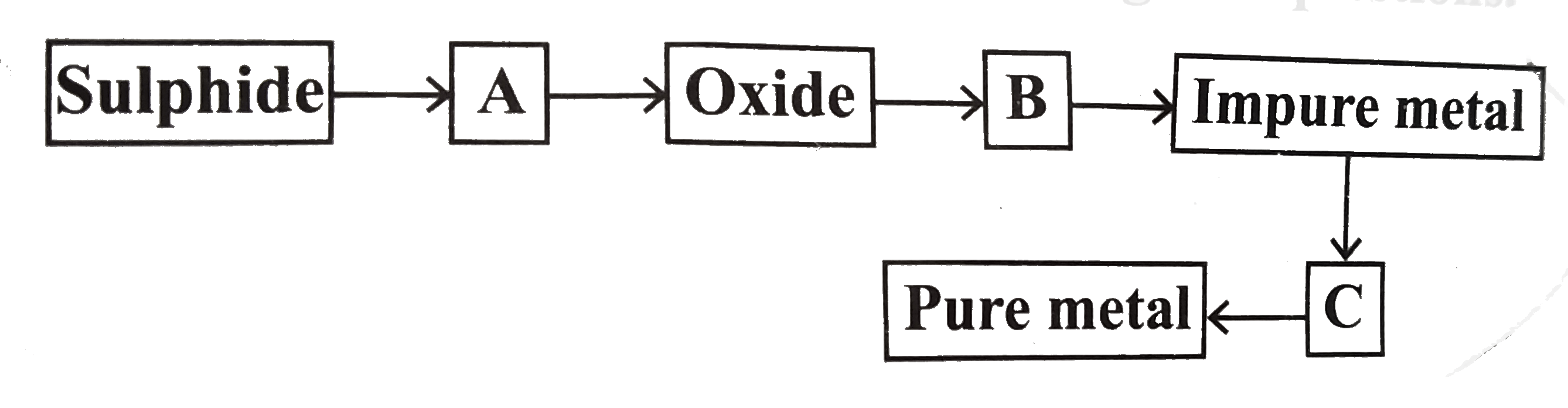
- From the following flowsheet for the extraction of pure metal, answer ...
Text Solution
|
- From the following flowsheet for the extraction of pure metal, answer ...
Text Solution
|
- From the following flowsheet for the extraction of pure metal, answer ...
Text Solution
|
- From the following flowsheet for the extraction of pure metal, answer ...
Text Solution
|
- From the following flowsheet for the extraction of pure metal, answer ...
Text Solution
|
- From the following flowsheet for the extraction of pure metal, answer ...
Text Solution
|
- The Ellingham diagram for a number of metallic sulphides is reproduced...
Text Solution
|
- The Ellingham diagram for a number of metallic sulphides is reproduced...
Text Solution
|
- The Ellingham diagram for a number of metallic sulphides is reproduced...
Text Solution
|
- Questions given below are based on the given diagram for extractive me...
Text Solution
|
- Free enegry , G = H - TS, is state function that indicates whther a re...
Text Solution
|
- Questions given below are based on the given diagram for extractive me...
Text Solution
|
- Questions given below are based on the given diagram for extractive me...
Text Solution
|
- Questions given below are based on the given diagram for extractive me...
Text Solution
|
- At high temperature, carbon reacts with water to produce a mixture of ...
Text Solution
|
- A overset("Calcination")rarr CaO + MgO + underset("Colourless gas")((B...
Text Solution
|
- A overset("Calcination")rarr CaO + MgO + underset("Colourless gas")((B...
Text Solution
|
- A overset("Calcination")rarr CaO + MgO + underset("Colourless gas")((B...
Text Solution
|
- A overset("Calcination")rarr CaO + MgO + underset("Colourless gas")((B...
Text Solution
|
- A overset("Calcination")rarr CaO + MgO + underset("Colourless gas")((B...
Text Solution
|