A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Multiple Correct)|43 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercise (Single Correcttype)|106 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exs 1.1 (True Orfalse Statement)|19 VideosELECTROCHEMISTRY
CENGAGE CHEMISTRY ENGLISH|Exercise Archieves Subjective|35 VideosGRIGNARD REAGENTS AND ORGANOMETALLIC REAGENTS
CENGAGE CHEMISTRY ENGLISH|Exercise Exercises Archives (Linked Comprehension)|1 Videos
Similar Questions
Explore conceptually related problems
CENGAGE CHEMISTRY ENGLISH-GENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS-Exercises (Linked Comprehension)
- Extraction of copper is done using copper pyrites. After roasting, th...
Text Solution
|
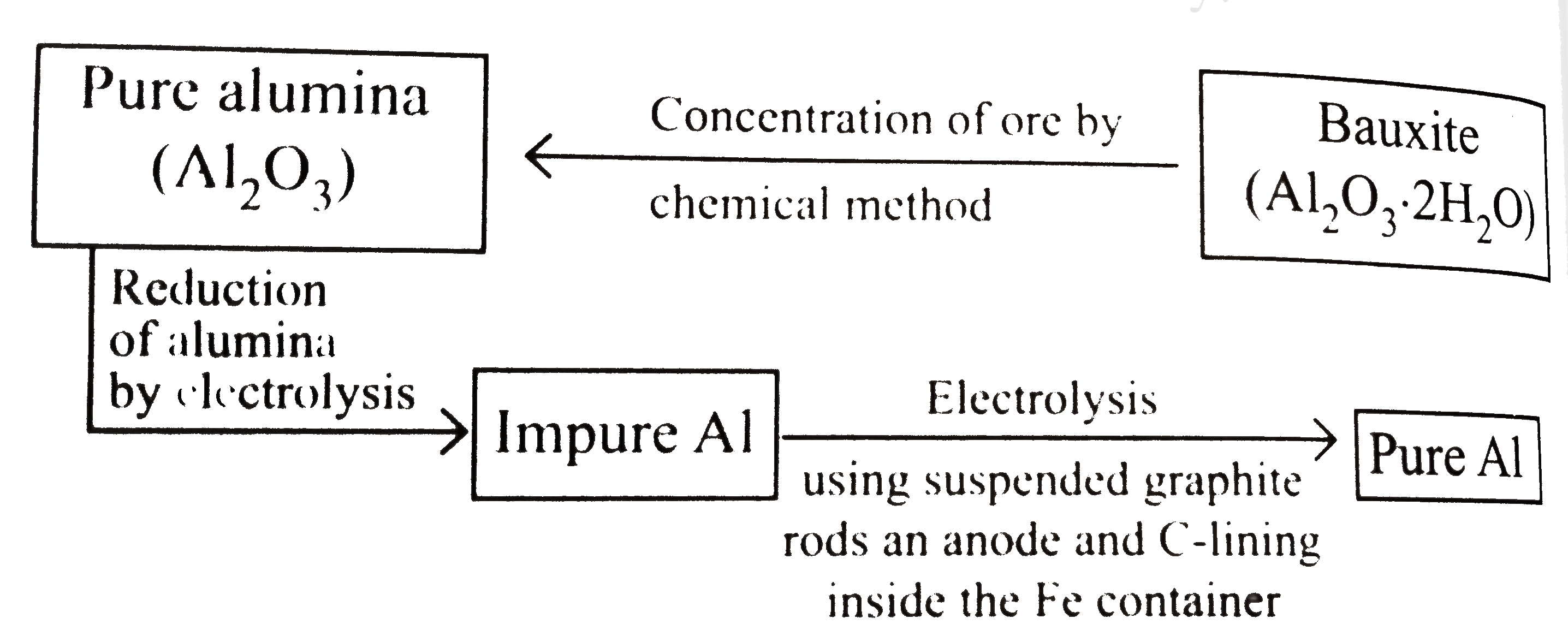
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Extraction of aluminium can be understood by : Electrolytric redu...
Text Solution
|
- Roasting is a process in which the ore (mostly sulphide) is heated str...
Text Solution
|
- Roasting is a process in which the ore (mostly sulphide) is heated str...
Text Solution
|
- Roasting is a process in which the ore (mostly sulphide) is heated str...
Text Solution
|
- Lead obtained form galena (PbS) by air reduction or carbon reduction p...
Text Solution
|
- Lead obtained form galena (PbS) by air reduction or carbon reduction p...
Text Solution
|
- Lead obtained form galena (PbS) by air reduction or carbon reduction p...
Text Solution
|
- Lead obtained form galena (PbS) by air reduction or carbon reduction p...
Text Solution
|
- Lead obtained from galena ore (PbS) by air reduction or carbon reducti...
Text Solution
|
- Lead obtained from galena ore (PbS) by air reduction or carbon reducti...
Text Solution
|
- Lead obtained from galena ore (PbS) by air reduction or carbon reducti...
Text Solution
|
- A is.
Text Solution
|
- B is :
Text Solution
|
- C is.
Text Solution
|
- E is.
Text Solution
|
- F is.
Text Solution
|